Objective:
1. To determine the extent of solvolysis of ammonium borate in water by calorimeter
Results and calculation:
Part I: Mixture of sodium hydroxide, NaOH and hydrochloric acid, HCl
| Time taken from beginning, t (s) | Temperature, T (°C) |
| 0.0 | 26.0 |
| 0.5 | 27.0 |
| 3.5 | 28.0 |
| 5.5 | 28.5 |
| 7.5 | 29.0 |
| 10.5 | 29.0 |
| 12.0 | 29.5 |
| 14.5 | 29.5 |
| 16.5 | 29.5 |
| 19.5 | 30.0 |
| 22.0 | 30.0 |
| 25.0 | 30.0 |
| 29.5 | 30.0 |
| 32.0 | 30.0 |
| 34.0 | 30.0 |
| 94.0 | 30.0 |
| 154.0 | 30.0 |
| 214.0 | 30.0 |
| 274.0 | 30.0 |
| 324.0 | 30.0 |
Graph 1 Temperature of mixture against time taken from beginning
| Initial temperature, Ti (°C) | 26.0 |
| Final temperature, Tf (°C) | 30.0 |
| Change of temperature, ΔT (°C) | 4.0 |
Part II: Mixture of sodium hydroxide, NaOH and boric acid, H3BO3
Table 2.1 The temperature of mixture and the time taken from beginning
| Time taken from beginning, t (s) | Temperature, T (°C) |
| 0.0 | 26.0 |
| 5.5 | 27.0 |
| 8.5 | 28.0 |
| 13.0 | 28.0 |
| 14.0 | 28.0 |
| 15.0 | 28.0 |
| 24.0 | 28.5 |
| 32.0 | 29.0 |
| 44.5 | 29.0 |
| 58.0 | 29.0 |
| 62.0 | 29.0 |
| 69.0 | 29.0 |
| 129.0 | 29.0 |
| 189.0 | 29.0 |
| 249.0 | 29.0 |
| 309.0 | 29.0 |
| 369.0 | 29.0 |
Graph 2 Temperature of mixture against time taken from beginning
| Initial temperature, Ti (°C) | 26.0 |
| Final temperature, Tf (°C) | 29.0 |
| Change of temperature, ΔT (°C) | 3.0 |
Part III: Mixture of ammonia, NH4OH and hydrochloric acid, HCl
Table 3.1 The temperature of mixture and the time taken from beginning
| Time taken from beginning, t (s) | Temperature, T (°C) |
| 0.0 | 25.0 |
| 2.0 | 26.5 |
| 5.0 | 27.0 |
| 6.0 | 28.5 |
| 8.0 | 29.0 |
| 10.5 | 29.0 |
| 12.5 | 29.0 |
| 15.0 | 29.0 |
| 17.5 | 29.5 |
| 20.0 | 29.5 |
| 23.0 | 29.5 |
| 26.0 | 30.0 |
| 29.5 | 30.0 |
| 33.0 | 30.0 |
| 39.0 | 30.0 |
| 99.0 | 30.0 |
| 159.0 | 30.0 |
| 219.0 | 30.0 |
| 279.0 | 30.0 |
| 339.0 | 30.0 |
Graph 3 Temperature of mixture against time taken from beginning
| Initial temperature, Ti (°C) | 25.0 |
| Final temperature, Tf (°C) | 30.0 |
| Change of temperature, ΔT (°C) | 5.0 |
Part IV: Mixture of ammonia, NH4OH and boric acid, H3BO3
| Time taken from beginning, t (s) | Temperature, T (°C) |
| 0.0 | 25.0 |
| 3.5 | 26.0 |
| 7.0 | 27.0 |
| 11.5 | 27.0 |
| 15.5 | 27.0 |
| 18.0 | 27.0 |
| 22.5 | 27.0 |
| 27.5 | 27.0 |
| 33.0 | 27.0 |
| 37.5 | 27.0 |
| 42.0 | 27.0 |
| 47.5 | 27.0 |
| 52.5 | 27.0 |
| 88.0 | 27.0 |
| 148.0 | 27.0 |
| 208.0 | 27.0 |
| 268.0 | 27.0 |
| 328.0 | 27.0 |
Graph 4 Temperature of mixture against time taken from beginning
| Initial temperature, Ti (°C) | 25.0 |
| Final temperature, Tf (°C) | 27.0 |
| Change of temperature, ΔT (°C) | 2.0 |
Determination of limiting agent in each part
In the reaction for part I, the limiting agent is calculated as shown below:

Therefore, limiting agent in the reaction is NaOH solution.
The limiting agent in each part is determined by using the method as shown in Part I.
Determination of heat capacity of calorimeter
From the enthalpy change, it can be expressed in the term of
-q = (msolution)(csolution)(ΔT) + (ccalorimeter )( ΔT)
-(-ΔH )(n) = (msolution)(csolution)(ΔT) + (ccalorimeter )( ΔT)
Since -ΔH = -q / n, and ΔH is negative because neutralization is an exothermic reaction.
Assumptions:
Specific heat capacity of calorimeter = 1.0 cal °C-1 g-1
Density of solution = 1g cm-3 (125ml solution = 125g solution)
Part I
In part I, the enthalpy change of a strong base and a strong acid is -13.36 kcal mol-1.
Number of moles of reaction = 0.04375 mol since limiting agent has 0.04375 mol.
Let ΔH1 be the enthalpy change of neutralization reaction of part I.
-(-ΔH1 )(n) = (msolution)(csolution)(ΔT) + (ccalorimeter )( ΔT)
-(-13.36x103 cal mol-1)(0.04375mol) = (125g)( 1.0 cal °C-1 g-1)(4°C) + (ccalorimeter )(4°C)
ccalorimeter = 21.125g °C-1
Determination of enthalpy change of each reaction
Part II
Based on part I, ccalorimeter = 21.125 cal °C-1, ΔT = 3°C
Number of moles of reaction = 0.04375 mol since limiting agent has 0.04375 mol.
Let ΔH2 be the enthalpy change of neutralization reaction of part II.
-(-ΔH2 )(n) = (msolution)(csolution)(ΔT) + (ccalorimeter )( ΔT)
-(-ΔH2)(0.04375mol) = (125g)( 1.0 cal °C-1 g-1)(3°C) + (21.125 cal °C-1)(3°C)
ΔH2 = 10.02 kcal mol-1
Since neutralization is an exothermic process, so ΔH2 = -10.02 kcal mol-1.
Part III
Based on part I, ccalorimeter = 21.125 cal °C-1, ΔT = 5°C
Number of moles of reaction = 0.04375 mol since limiting agent has 0.04375 mol.
Let ΔH3 be the enthalpy change of neutralization reaction of part III.
-(-ΔH3 )(n) = (msolution)(csolution)(ΔT) + (ccalorimeter )( ΔT)
-(- ΔH3)(0.04375mol) = (125g)( 1.0 cal °C-1 g-1)(5°C) + (21.125 cal °C-1)(5°C)
ΔH3 = 16.7 kcal mol-1
Since neutralization is an exothermic process, so ΔH3 = -16.7 kcal mol-1. However, this value is not valid due to certain error. The enthalpy change of the neutralization reaction between a weak base and a strong acid should be less than the reaction between strong acid and strong base.
Part IV
Based on part I, ccalorimeter = 21.125 cal °C-1, ΔT = 2°C
Number of moles of reaction = 0.04375 mol since limiting agent has 0.04375 mol.
Let ΔH4 be the enthalpy change of neutralization reaction of part IV.
-(-ΔH4 )(n) = (msolution)(csolution)(ΔT) + (ccalorimeter )( ΔT)
-(- ΔH4)(0.04375mol) = (125g)( 1.0 cal °C-1 g-1)(2°C) + (21.125 cal °C-1)(2°C)
ΔH4 = 6.68 kcal mol-1
Since neutralization is an exothermic process, so ΔH4 = -6.68 kcal mol-1.
Determination of enthalpy change of dissociation of H3BO3 and NH3
Assumption: The reaction between weak base and weak acid goes to completion.
By using the ΔH’s value of obtained,
ΔH4 = ΔH2 + ΔH3 – ΔH1
ΔH4 = -10.02 kcal mol-1 + (-16.7 kcal mol-1) – (-13.36kcal mol-1)
ΔH4 = -13.36 kcal mol-1
The enthalpy change of neutralization between a weak base and a weak acid is -13.36 kcal mol-1. By comparing to the value calculated, ΔH4 = -6.68 kcal mol-1 (from part IV), this value is bigger. According to the reaction between weak base and weak acid, this value is not valid because the expected value should be less than the value obtained in part I, part II and part III. Since the value of ΔH3 is not valid, therefore the value obtained by using ΔH4 = ΔH2 + ΔH3 – ΔH1 is no longer valid.
Determination of fraction of the salt ammonium borate reacts with water
Let a be the fraction of ammonium borate salt reacts with water
Ksolv = equilibrium constant of solvolysis
a = 1 – a’ = 1 – (ΔH / ΔH4)
K = a2 / (1-a)2
Based on the formula given above, the fraction of ammonium borate salt reacts with water and equilibrium constant of solvolysis were calculated in each part. 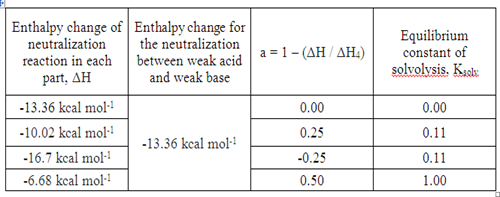
For ΔH = -16.7 kcal mol-1, the value of fraction of the salt ammonium borate reacts with water is negative value which is -0.25. This is an invalid value. Hence, the value of equilibrium constant of solvolysis is not valid too.
Determination of the dissociation constant, Ka for boric acid
From the previous part, the equilibrium constant of solvolysis for part IV is 1.00, and the value of Kw = 1.0 x 10-14 and Kb = 1.75 x 10-5. Therefore, the value of Ka of boric acid is calculated as shown below:
1.00 = 1.0 x 10-14 / (Ka)(1.75 x 10-5)
Ka = 5.71 x 10-10
According to the journal article, Perelygin, Yu. P. & D. Yu. Chistyakov (2006). Boric acid. Russian Journal of Applied Chemistry, 79(12), 2041-2042. doi: 10.1134/S1070427206120305. (Dissociation constant of Ka is 7.3 x 10-10)
The value obtained experimentally is 5.71 x 10-10 which shows a deviation with the value of Ka obtained via journal article.





No comments:
Post a Comment