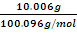Objectives:
1. To study the physical and chemical properties of alcohols
2. To identify the two unknown liquids from their experimental observations
3. To study the difference between primary alcohols, secondary alcohols and tertiary alcohols.
Introduction:
The hydrocarbon chains that attached with a hydroxyl group, OH- to a carbon atom are known as alcohols. If the carbon atom is bonded to three hydrogen in addition to the OH-, the alcohol is called methanol. Methanol, CH3OH is the most simple alcohol molecule. The category of the alcohol is classified as three groups which are primary (1 ) alcohol, secondary (2 ) alcohols and tertiary (3 ) alcohol. If the alcohol bonded to one alkyl group, the alcohol is primary alcohol. The secondary alcohol is defined as the alcohol which one of the carbons is bonded to two alkyl groups and one hydrogen atom. If one of the carbons in alcohol is bonded to three alkyl groups is called tertiary alcohol.
All these alcohols share some similar characteristics but other characteristics are different owing to their different molecular structures. For physical properties, the size of alcohol determines its boiling point. Usually, the larger the size of the alcohol, the higher the boiling point. This is because the bigger the size of the molecules, the stronger the Van der Waals force between the alcohol molecules. So, more heat energy is needed to be absorbed in order to break down the intermolecular force between each alcohol molecules. Hence, the boiling point of the alcohols increases with the size of the alcohols. The solubility of the alcohol is depends on the size of molecules. Small alcohols are water soluble because the hydroxyl group can form hydrogen bond with water molecules. But, as the size of the alkyl group increases, the solubility of alcohol in water decreases as the hydrophobic property of alcohol increases. For example, if the carbon molecules in alcohol more than six per molecule, the particular alcohol definitely are not soluble in water. This is the result of the alkyl group disrupting the hydrogen bond among the water molecules. If the disruption becomes larger enough, the water molecules will repel the alcohol molecules effectively to reestablish hydrogen bonding.
The classification of an alcohol as primary, secondary or tertiary (see above) affects the chemical properties of the alcohols. Due to their different classes, the alcohols may give out different chemical properties when they react with the same compounds. Based on their chemical properties, we are able to differentiate among the classes of alcohols. Generally, Lucas test and chromic acid test is the two common tests that we always use to distinguish and categorize the classes of alcohols.
Lucas test
ZnCl2 |
R-OH + HCl ------------------> R-Cl + H2O
Chromic acid test
Chromic acid is a strong oxidizing agent which uses to oxidize the alcohols. This test is based on the reduction of chromium (VI) ions to chromium (III) ion. When chromic acid reacts with alcohols, the change in colour of the solution from red-brown to green is a positive test. Primary alcohols are oxidized to carboxylic acid by chromic acid. The Cr6+ in the chromic acid which is red-brown is reduced to green Cr3+; secondary alcohols are oxidized to ketones by the reagent with the same colour change. Tertiary alcohols are not oxidized at all by the chromic acid. Hence, this reaction can be used to distinguish tertiary alcohols form primary and secondary alcohols.For tertiary alcohols, the alcohols would not be oxidized by the reagent. Hence, this test is used to distinguish the tertiary alcohols from primary and secondary alcohols.
Reaction with sodium metal
The acidic properties of alcohol can be shown by adding the sodium metal into alcohol. The alcohols are weak acids when they react with sodium metal. The hydroxyl group can act as a porton donor to form an alkoxide ion. Alkoxide ions dissolved in alcohol are strong bases which can be prepared by the reaction of an alcohol with sodium metal. Hydrogen gas is released by the reaction.
2 R-OH + 2 Na -------> 2 R-O-Na+ + H2
The hydrogen gas can be collected and tested by using a burning wooden splinter. A pop sound will be produced.
Apparatus: test tube, measuring cylinder, droppers
Materials: ethanol (C2H5OH), isopropyl alcohol (C3H7OH), t-butyl alcohol (C4H9OH), Lucas reagent (mixture of concentrated HCl and ZnCl), chromic acid (H2CrO4), sodium metal
Procedure:
A. Solubility of alcohols
1. In three separate dry test tubes, ethanol, isopropyl alcohol and t-butyl alcohol are added into each test tube.
2. Water is added to each tubes, the contents are mixed and observed.
3. The results are recorded in a table.
4. The above procedures are repeated with two unknown liquids and observations are being made.
B. Lucas test
1. Ethanol, isopropyl alcohol and t-butyl alcohol are added into separate dry test tubes and Lucas reagent is added at room temperature.
2. The tubes are closed with a cork, the tubes are shaken and the length of time it takes for the mixture to become cloudy or separate two layers.
3. The results are recorded.
4. The above procedures are repeated with the two unknown liquids and observations are being made.
C. Chromic acid test
1. Ethanol, isopropyl alcohol and t-butyl alcohol are added into separate dry test tubes.
2. A small piece of sodium metal is added and any reactions occur are noted.
3. The step 1 and step 2 are repeated with two unknown liquids and any observations are made.
Results:
Part A
Table 1 The solubility of alcohols and their observations
Alcohols | Observation | Reaction equations(if any) | Deduction/conclusion/discussion |
Ethanol | Soluble in water to form colourless solution | C2H5OH (aq) + H2O (l) ---> C2H5O- (aq)+ H+(aq) | Soluble in water |
Isopropyl alcohol | Soluble in water to form colourless solution | C3H7OH (aq) + H2O (l) ---> C3H7O- (aq) + H+ (aq) | Soluble in water |
t-butyl alcohol | Soluble in water to form colourless solution | C4H9OH (aq) + H2O (l) -----> C4H9O- (aq) + H+ (aq) | Soluble in water |
Unknown A | Soluble in water to form colourless solution | - | Soluble in water |
Unknown B | Soluble in water to form colourless solution | - | Soluble in water
|
Part B
Table 2 The reaction between alcohols and Lucas Reagent
Alcohols | Observation | Reaction equations(if any) | Deduction/conclusion/discussion |
Ethanol | Yellowish solution remains unchanged after heating. |
| No reaction between ethanol and Lucas reagent. |
Isopropyl alcohol | Yellowish solution remains unchanged within 10 minutes, but turns into cloudy solution after heating. | C3H7OH (aq) + HCl (aq) -----> C3H7Cl (aq) + H2O (l) | The reaction between isopropyl alcohol and Lucas reagent occur after heating for 10 minutes. |
t-butyl alcohol | Cloudy solution is formed immediately and two layers are formed. Upper layer is clear while lower layer is cloudy. | C4H9OH (aq) + HCl (aq) C4H9Cl (aq) + H2O (l) | The reaction between t-buytl alcohol and Lucas reagent is instant reaction. |
Unknown A | Yellowish solution remains unchanged within 10 minutes, but turns into cloudy solution after heating. |
| Unknown A is isopropyl alcohol because it has the similar reaction with it. |
Unknown B | Cloudy solution is formed immediately and two layers are formed. Upper layer is clear while lower layer is cloudy. |
| Unknown B is t-butyl alcohol because it has the same reaction with it. |
Part C
Table 3 The reaction of alcohols with chromic acid
Alcohols | Observation | Reaction equations(if any) | Deduction/conclusion/discussion |
Ethanol | The solution turns from colourless to green | 3 CH3CH2OH + 2 CrO4- + 10 H+ à 3 CH3CHO + 2 Cr3+ +8 H2O
| Ethanol is oxidized by the chromic acid. |
Isopropyl alcohol | Two layers are formed. Upper layer is green while lower layer is black. | C3H7OH (aq) + H2CrO4(aq) -----> C2H6CO (aq) + Cr2(SO4)3(aq) + H2O(l)3 (CH3)2CHOH + 2 CrO4- + 10 H+ à3 (CH3)2CO + 2 Cr3+ +8 H2O
| Isopropyl alcohol is oxidized by the chromic acid. |
t-butyl alcohol | Two layers are formed. Then, the precipitate dissolves in solution to become reddish brown solution. | - | No reaction. |
Unknown A | Two layers are formed. Upper layer is green while lower layer is black. |
| Unknown A is isopropyl alcohol because it has the similar reaction with isopropyl alcohol. |
Unknown B | Two layers are formed. Then, the precipitate dissolves in solution to become reddish brown solution. |
| Unknown B is t-butyl alcohol because it has the similar reaction with t-butyl alcohol. |
Part D
Alcohols | Observation | Reaction equations(if any) | Deduction/conclusion/discussion |
Ethanol | Bubbles of colourless gas released quickly. A pop sound is produced when tested with burning wooden splinter. | 2C2H5OH (aq) + 2Na(s) ------> 2C2H5ONa (aq) + H2(g) | Many of hydrogen gas is being produced from the reaction between ethanol and sodium metal. |
Isopropyl alcohol | Bubbles of colourless gas released slowly. A pop sound is produced when tested with burning wooden splinter. | 2C3H7OH (aq) + 2Na(s) -----> 2C3H7ONa (aq) + H2(g) | Small amount of hydrogen gas is being produced from the reaction between isopropyl alcohol and sodium metal. |
t-butyl alcohol | Bubbles of colourless gas released very slowly. A pop sound is produced when tested with burning wooden splinter. | 2C4H9OH (aq) + 2Na (s) ------> 2C4H9Ona (aq) + H2 (g) | Less hydrogen gas is being produced from the reaction. |
Unknown A | Bubbles of colourless gas released slowly. A pop sound is produced when tested with burning wooden splinter. |
| Unknown A is isopropyl alcohol because it has the similar reaction with isopropyl alcohol. |
Unknown B | Bubbles of colourless gas released very slowly. A pop sound is produced when tested with burning wooden splinter. |
| Unknown B is t-butyl alcohol because it has the similar reaction with t-butyl alcohol. |
Discussion:
This is because all of them, ethanol, isopropyl alcohol and t-butyl alcohol are short alkyl chain alcohols. Alcohols can soluble in water is because of the presence of the hydroxyl group (-OH) in the compounds. The hydroxyl group can form hydrogen bond with the water molecules and thus make it soluble in water.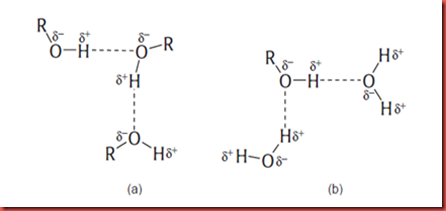 The solubility of alcohols in water are always depends on their structure and size. When the sizes of alcohols increase, the solubility of alcohols in water will decrease. This is because the bulky groups are highly hydrophobic and tend to block the water molecule from nearing alcohol and stabilize it. This is the result of the alkyl group disrupting the hydrogen bond among the water molecules. If the disruption becomes larger enough, the water molecules will repel the alcohol molecules effectively to reestablish hydrogen bonding. Usually, the number of carbon per molecule is more than six are not soluble in the water.
The solubility of alcohols in water are always depends on their structure and size. When the sizes of alcohols increase, the solubility of alcohols in water will decrease. This is because the bulky groups are highly hydrophobic and tend to block the water molecule from nearing alcohol and stabilize it. This is the result of the alkyl group disrupting the hydrogen bond among the water molecules. If the disruption becomes larger enough, the water molecules will repel the alcohol molecules effectively to reestablish hydrogen bonding. Usually, the number of carbon per molecule is more than six are not soluble in the water.
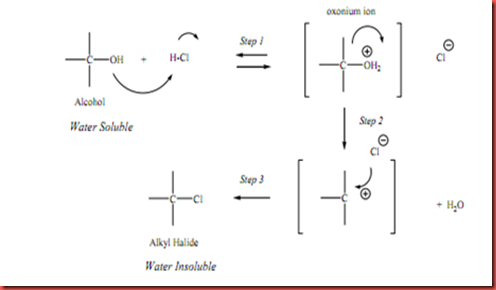
Based on the properties of solubility of alcohols in water, this information is not enough for us to differentiate clearly the classes of alcohols which are being used in the experiment. So, Lucas test is used to distinguish among the primary, secondary, and tertiary alcohols. For Lucas test, the formation of two layers (aqueous layer and cloudy layer) is known as a positive test. The second layer (cloudy layer) formed is alkyl chloride which is insoluble in the aqueous solution because all the alkyl halides molecules are insoluble in the water. The alkyl chloride produced from the reaction is not water soluble and causes cloudiness (emulsion) to form in the aqueous solution. When ethanol is added with Lucas reagent, the yellowish solution is still remains the same before and after heating. This is because primary alcohol does not react with Lucas reagent. Besides, isopropyl alcohol does not react with Lucas reagent before heating but it does turns to cloudy solution after heating for 10 minutes. The reason is the reaction between Lucas reagent and secondary alcohol is slow. For tertiary alcohol (t-butyl alcohol), the reaction of alcohol with Lucas reagent is very fast which can be known as an instant reaction. The reaction that takes place on the Lucas test is a SN1 nucleophilic substitution. The alcohols with the properties of generating a stable carbocation intermediates will undergo the particular reaction. The OH group of the alcohol attracts the H in hydrochloric acid to form oxonium ion and leave the group (form water). The carbocation intermediate is formed and tends to react with Cl- (nucleophile) to produce the alkyl halide product. The mechanism of SN1 nucleophilic substitution is shown as diagram below:
The purpose of chromic acid test used in this experiment is to distinguish the primary and secondary alcohols from the alcohols group. In the reaction between alcohols and chromic acid, the chromic acid is being reduced which the chromium (VI) ions, Cr6+ reduced to become chromium (III) ion, Cr3+. The positive test for chromic acid is represented by the change in colour from orange to green-blue. In the test tube with ethanol, the colourless alcohol is turned to green solution because the chromium (IV) ions, Cr6+ are being reduced by ethanol. In the test tube containing isopropyl alcohol, two layers of colour are formed. The upper layer is green whereas the lower layer is black. This is shows that the isopropyl reacted with the chromic acid due to the secondary alcohol is readily to be oxidized. The black layer actually is the dark blue colour, it is hard to differentiate because the light is not enough in the laboratory. The t-butyl alcohol would not oxidized by the chromic acid since the tertiary alcohol is a highly oxidized alcohol. This is shown by the formation of reddish brown precipitate. The chemical equation for the reaction is shown as below:
Ethanol: 3 CH3CH2OH + 2 CrO4- + 10 H+ à 3 CH3CHO + 2 Cr3+ +8 H2O
Isopropyl alcohol: 3 (CH3)2CHOH + 2 CrO4- + 10 H+ à3 (CH3)2CO + 2 Cr3+ +8 H2O
The general mechanism for the reaction is shown below:
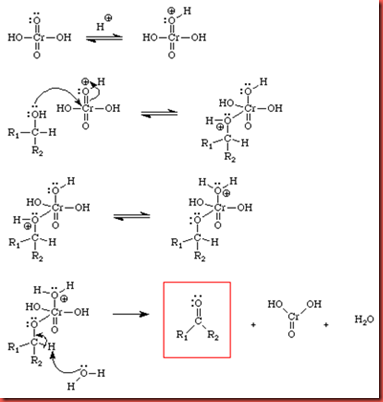 Hydrogen ions will be attracted to one of the oxygen atom in the chromic acid that is double bonded to chromium. The lone pair electrons from hydroxyl group of alcohols will then attack the chromium ion and form a chromate intermediate. The hydrogen on the hydroxyl group of alcohols will then leave as hydrogen ion and combine with the oxygen atom that is previously attacked by a hydrogen ion. A water molecule will come and attack α-hydrogen on the alcohol and produce hydronium ion, forming C=O double bond. A water molecule will leave the chromate intermediate and the carbonyl product is formed. Since the oxidation of alcohol requires at least one hydrogen atom to be presence on α-carbon, thus tertiary alcohol cannot be oxidized because it does not have the α-hydrogen. This explains why t-butyl alcohol does not change the color of the chromic acid which is red brown to green. The precipitate formed at the beginning may be because of the formation of chromium trioxide precipitate.
Hydrogen ions will be attracted to one of the oxygen atom in the chromic acid that is double bonded to chromium. The lone pair electrons from hydroxyl group of alcohols will then attack the chromium ion and form a chromate intermediate. The hydrogen on the hydroxyl group of alcohols will then leave as hydrogen ion and combine with the oxygen atom that is previously attacked by a hydrogen ion. A water molecule will come and attack α-hydrogen on the alcohol and produce hydronium ion, forming C=O double bond. A water molecule will leave the chromate intermediate and the carbonyl product is formed. Since the oxidation of alcohol requires at least one hydrogen atom to be presence on α-carbon, thus tertiary alcohol cannot be oxidized because it does not have the α-hydrogen. This explains why t-butyl alcohol does not change the color of the chromic acid which is red brown to green. The precipitate formed at the beginning may be because of the formation of chromium trioxide precipitate.
The last test in this experiment is the reaction of sodium metal with alcohols. All the different classed of the alcohol are able to react with the sodium metal since all of them have OH group. According to the Lewis Bronsted Theory, a Bronted acid is a proton donor. In this case, alcohol acts as a proton donor which donates H+ into the solution while the sodium metal acts as a strong base. A strong base can deprotonates the alcohol to yield an alkoxide ion (R-O). The H in OH group will be substituted by sodium ion to form sodium alkoxide, R-O-Na+. The reaction between an acid and an active metal in group I element definitely will produce hydrogen gas as the product as the metallic sodium reduces proton to form hydrogen gas. The evidence is shown by the release of bubbles of colourless gas from the reaction between sodium metal and alcohol. The release of hydrogen gas can be collected and tested with a burning wooden splinter. A pop sound will be produced as the hydrogen gas is an explosive gas. The chemical equation of alcohols and sodium metal is shown as the diagram 1 below:
2C2H5OH (aq) + 2Na(s) 2C2H5ONa (aq) + H2 (g)
2C3H7OH (aq) + 2Na(s) 2C3H7Ona (aq) + H2 (g)
2C4H9OH (aq) + 2Na (s) 2C4H9Ona (aq) + H2 (g)
Diagram 1 The reaction between alcohols with different classes with sodium metal
The acidity of the alcohol can be determined by the rate of gas evolution in this experiment. The more the gas released from the reaction, the more acidic the properties of alcohol. The acidity of alcohols decreases as the number of carbon in bulky alkyl group that bonded to OH group increases. Since alkyl group is electron releasing group, the more bulky the alkyl group, the stronger the electron donor effect. Thus, the negative charge density on the O atom in and proton are less readily to be released. The second reason is the bulky groups are highly hydrophobic and tend to block the water molecule from nearing alcohol and stabilize it. The alcohols are arranged in order of increasing acidity of alcohol as below:
t-butyl alcohol < isopropyl alcohol < ethanol
Acidity increasing
![clip_image004[1] clip_image004[1]](http://lh6.ggpht.com/_VE77yZI3fI0/TcSp4-uC4FI/AAAAAAAAAWQ/TL_Gq2LJz7M/clip_image004%5B1%5D_thumb%5B1%5D.jpg?imgmax=800)