Objectives:
1. To produce methyl benzoate by esterification
2. To learn the reaction mechanism involved in esterification
3. To demonstrate how an ester can be made by the interaction of a carboxylic acid and an alcohol with the presence of a sulfuric acid catalyst.
Introduction:
The ester group is an important functional group that can be synthesized in a number of different ways. The low molecular-weight esters have very pleasant odours and indeed are major components of the flavour and odour aspects of a number of fruits. Although the natural flavour may contain nearly a hundred different compounds, single esters approximate the natural odours and are often used in the food industry for artificial flavours and fragrances.
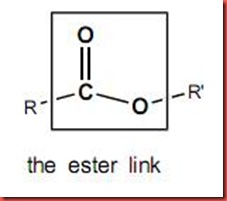
The esterification is a reaction between an alcohol and a carboxylic acid or a carboxylic acid derivative, water and ester will be formed as products in this process under reflux. In the chemical structure of carboxylic acid, R-COOR’, where R and R' are either alkyl or aryl groups. As shown in the diagram below, the esterification is also known as condensation process which water is produced.
In this case, each of the carboxylic acid will contributes hydroxyl group while each alcohol will contribute hydrogen atom to form a water molecule. So, the reaction involves the removal of water once the ester is formed. The ester linkage will appear as the bond that connected the one carboxylic acid and one alcohol in the ester molecule as shown as the following:
Generally, an acid will be used to act as a catalyst in esterification process.
The esterification is a reversible process which the equilibrium between reactants and products will be reached. The opposite of the esterification reaction is called hydrolysis. The addition of water to the ester link will cause breaking apart of the ester into their parent carboxylic acid and alcohol. The hydrolysis also requires the presence of a catalyst (either acid or base). The esterification is a slow process. The main reason is due to the water produced in the esterification will be used back in the hydrolysis which converts the ester to form parent alcohol and carboxylic acid in the reaction. As the ester is start to form in the reaction but at the same time the hydrolysis start to begin. An equilibrium is finally attained, all of the related reactants and products will present in the mixture formed via esterification and hydrolysis.
The mechanism of the formation of ester under acidic condition might be follows this steps below:
|
However, the esterification only can be applied by using simple alcohol and carboxylic acid in acidic condition. In another word, the long chain alcohol cannot be used to react with carboxylic acid. We can use another method to produce ester by using carboxylic acid reacts with haloalkane in a basic condition. But the same problem may be encountered at the end, this method is only limited to the primary alkyl halides. So, in order to utilize the maximum ester, an alternative for the preparation of esters is to treat the alcohol with a reactive carboxylic acid derivative. For example, carboxylic acid anhydrides or chloride can be used.
(RCO)2-O + R’’OH -----> RCOOR’’ + RCO2H
RCOCl + R’’OH -------> RCOOR’’ +HCl
These reaction is irreversible and they react rapidly especially when catalyzed by a strong acid.
Apparatus:
Round bottomed flask (250ml), Liebig condenser, separating funnel, Bunsen burner. Thermometer
Materials:
Chloroform (trichloromethane), anhydrous sodium sulphate, benzoic acid, methanol, conc. sulphuric acid, anti bumping granules
Procedure:
1. Benzoic acid is placed into a 250ml beaker and then methanol is added .
2. Concentrated sulphuric acid is added whilst swirling the contents and washed with methanol.
3. Two or three anti-bumping granules are added to the mixture and fit in to the reflux set up.
4. The mixture is being refluxed for 1 hour, the mixture is poured into a separating funnel with 150ml of water after cooling.
5. The reaction flask is rinsed with chloroform two times and is added into the funnel.
6. The aqueous layer is being removed and water is used to wash the organic layer.
7. Anhydrous sodium sulphate is used to dry the solution and then is filtered out.
8. The organic solvent is distilled at three different ranges, which are 30-90°C, 91-190°C and 190°C above.
9. The ester is collected in a weighed flask and the distillation temperature range is noted.
10. The percentage of theoretical yield is calculated.
Results:
Weight of benzoic acid = 12.2017g
Weight of conical flask = 49.1914g
Weight of conical flask + weight of ester = 62.5712g
Weight of ester = 13.3798g
C6H5COOH + CH3OH <-----> C6H5COOCH3 + H2O
Number of moles of C6H5COOH = 12.2017g / 122.118 g mol-1
= 0.09992 mole
0.09992 mole of benzoic acid will produce 0.09992 mole of ester
Weight of C6H5COOCH3 = 0.09992 mole x 136.144 g/mol
= 13.6035 g
Percentage yield = 13.3798g/ 13.6035g x 100%
= 98.3556 %
Discussion:
Benzoic acid and methanol are used the reactants with the presence of sulphuric acid in this experiment. The acid catalysed reaction between benzoic acid and methanol may be represented as:
This esterification using the benzoic acid and methanol is known as Fisher esterification. The concentrated sulphuric acid is added as a catalyzed in this experiment. The purpose of using catalyze is to speed up the esterification because it is a slow process. Concentrated sulphuric acid also serve as another function which is used to protonates the carboxylic acid and hence this will initiate the reaction to start. The esterification between benzoic acid and methanol is favourable in acidic condition; as a result more ester can be formed in this experiment.
After added with the concentrated sulphuric acid, another portion of methanol is introduced into the mixture. The adding of methanol is to ensure all the carboxylic acid could be reacted completely during reflux. Furthermore, anti-bumping granules are added to promote smooth boiling and to prevent bumping of the solvent. As mention at the above, the esterification is slow and reversible process so it is distilled for one hour and hence more ester could be generated. The esterification mechanism is take place as the diagram 1 below:
Diagram 1
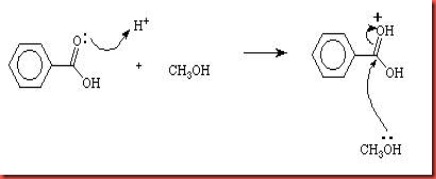
The dissociation of sulphuric acid will produce hydrogen ion which can be used to protonates the carbonyl group in benzoic acid. The carbonyl group is protonated reversibly and caused the positive charge of carbonyl group to be increased. Thus this increases the reactivity of carbonyl group towards nucleophile. The C-O double bond is broken in order to stabilize the OH+ group to form hydroxyl group in the benzoic acid molecule. The methanol acts as a nucleophile attacks the benzoic acid.
Diagram 2
In the diagram 2, the methanol successful attacked the carbonyl group to form a new C-O bond to the carboxyl group in the benzoic acid to form a tetrahedral intermediate. This is called nucleophilic addition. The oxygen atom in the carboxyl group in benzoic acid is more electronegative due to its lone pair electron. The lone pair electron in the particular electron attracts the hydrogen atom from the methanol to form oxonium ion. Now, the oxygen atom in the methanol becomes unstable and hence the C-H bond will tend to be broken down. The electron between the C-H bond will delocalise to the oxygen atom of the methanol. The formation of oxonium ion in the carboxyl group in benzoic acid tends to be released from the intermediate to form water. Eventually, another hydroxyl group will donate the lone pair electron to the attached oxygen atom to form a more stable intermediate.
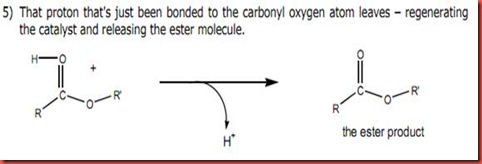
Diagram 3
The hydrogen atom in the ester intermediate will be attacked the acid in diagram 3 and the acid catalyze is regenerated. Thus, finally the methyl benzoate is formed.
During esterification, the hydrolysis process start to begin once the ester is being produced. The water produced in esterification is used back in the hydrolysis to hydrolyze the ester to form the carboxylic acid and alcohol. The process is a continuous reversible process until the equilibrium is reached. The hydrolysis process would be the following equation:
The hydrolysis process must under acidic or basic condition in order to break down the stable ester molecule. To avoid the hydrolysis process, the water could be removed from the mixture. According to Le Chatelier’s principle, the equilibrium position will shift to the product side if water is being removed. Another method to increase yield of ester is by adding more alcohol into the mixture. Hence, more ester could be generated when the amount of alcohol increased which shift the equilibrium to product side. The mechanism of hydrolysis is shown in the diagram 4 below:
Diagram 4
Water molecule acts as nucleophile to attack the carbonyl carbon of ester reversiblely. The C=O will be break and the oxygen atom attached to the carbonyl carbon form negative oxygen atom. The methoxy group tends to leave the tetrahedral intermediate to form a stable methoxide, so the negative oxygen atom donates the electron back to the carbonyl carbon. Finally, the methoxide acts as a nucleophile which attack the hydroxyl group bonded to carbonyl carbon and hence to form methanol. The oxygen atom now is negatively charge and it tends to get a proton from the environment. Thus benzoic acid is formed.
After reflux for one hour, the reaction mixture is introduced into the separating funnel with distilled water to carry out extraction. This is because the unreacted methanol can be dissolved in distilled water and hence it could be removed together with the aqueous layer. Small amount of the benzoic acid present in the mixture will dissolve in the water although it is highly insoluble in water. Distilled water can extracts the leftover of methanol and small amount of benzoic acid. Chloroform is added to extract the ester by dissolving the ester in the chloroform. After the aqueous layer is being removed, the anhydrous sodium sulphate is added as drying agent which will absorb water droplet and hence residual water can be removed completely.
Now, the residual ester still contains some methanol, benzoic acid and other side products. So, we use distillation to obtain the pure ester from the mixture. During distillation, the temperature of the distillate is kept constant at 63°C. This is because some of the methanol is still left in the mixture and its boiling point is around 65°C. So, the temperature remains at 63°C until all the methanol are completely distilled. For the second time of distillation, the temperature of distillate is keep changing in the range of 140°C to 180°C. This might be due to the mixture contain some impurities, so the temperature may fluctuate in the wider range of temperature. Another reason for the fluctuation of temperature may be side product formed during reflux is exist in the mixture. So, this contribute to the temperature to be fluctuated. The ester is being synthesized is 98.3556% but this figure is not reliable. The weight of the solution is very high due to the impurities present in the solution. The actual figure that an ester could be form is 60-70%, which according to the estimation of scientist.










![clip_image002[5] clip_image002[5]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEi0NHLWEhjUL-s8OW9lfZyo1yr0OcctiIl-kwTSzhCLDPRdEPs9qc0ZA8sUNIqoMwi8C7IVrowpardL7dMXAHs1pMajJbl8jEHa1G1XjFu8ts3UU5eAL7tpOHrxWrrG97TQmOyMQwbox0JM/?imgmax=800)




![clip_image031[5] clip_image031[5]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEhpeDmiIjpCA5PDKuGO7HK7ZGaeAoFLkHgTBSiGNftH0Xc177w08EIqqgsKQPND3r5RzA0Z1ziagQMwpTKp3Za5mSZJbDT8HKPfpHxfcELns3GQEYviK_DeXaTer9u3NgBTpY1tFPWGx026/?imgmax=800)
![clip_image033[5] clip_image033[5]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEjWkbZwuEgozcVT4jWfQEKgbgq34a3bFRKL1NfgjMwvUC2TVsyhwPN4bOb80Iz7FkPKREQnQR3Sa5gcx1s80JaAr0X80hHlT6uqkrt11MXxQn56hnqzmjDg_1YOfa-dw5VzpW576uPzv8wN/?imgmax=800)
![clip_image035[5] clip_image035[5]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEiPZfpRWpoIRCekwGOI23qPfS-HiN3-5z-guY3s2kvtH1Ws1ygfQS8GH0L2yjLSQbRwZ_-YHgYm8ZjekC1TFVQ55kePQYS94USj_uvfAow9julHvtplDGXci0JO80WyHp8FzHN-LRktRW_F/?imgmax=800)

