Objective:
1. To carry out a mixed aldol condensation reaction
2. To study the mechanism of aldol condensation reaction
Introduction:
The reaction of an aldehyde with a ketone employing sodium hydroxide as the base is an example of a mixed aldol condensation reaction, the Claisen-Schmidt reaction. The double mixed-aldol condensation reaction between acetone and benzaldehyde was carried out. Acetone has α-hydrogens (on both sides) and thus can be deprotonated to give a nucleophilic enolate anion. The alkoxide produced is protonated by solvent, giving a β-hydroxyketone, which undergoes base-catalyzed dehydration. The elimination process is particularly fast in this case because the alkene is stabilized by conjugation to not only the carbonyl but also the benzene. In this experiment, excess benzaldehyde such that the aldol condensation can occur on both sides of the ketone.
Dibenzalacetone is readily prepared by condensation of acetone with two equivalent of benzaldehyde. The aldehyde carbonyl is more reactive than that of the ketone and therefore reacts rapidly with the anion of the the ketone to give a β-hydroxyketone, which easily undergoes base catalyzed dehydration. Depending on the relative quantities of the reactants, the reaction can give either mono- or dibenzalacetone.
Dibenzalacetone is a fairly innocuous substance in which its spectral properties indicate why it is used in sun-protection preparations. In the present experiment, sufficient ethanol is present as solvent to readily dissolve the starting material, benzaldehyde and also the intermediate, benzalacetone. The benzalacetone once formed, can then easily to react with another mole of benzaldehyde to give the desired product in this experiment, dibenzalacetone.
Apparatus: Erlenmeyer flask, Buchner funnel, glass funnel, melting point apparatus, UV/Vis spectrometer, FTIR spectrometer
Materials: Benzaladehyde, acetone, sodium hydroxide, 95% ethanol, ethyl acetate, ice
Procedure:
1. 5g of NaOH was added to 25ml of H2O in an Erlenmeyer flask and the solution was swirled.
2. 25ml of 95% ethanol was added and the solution was allowed to come nearly to room temperature.
3. 2.9g of acetone and 10.5ml of benzaladehyde were added.
4. After 15 minutes of occasional swirling, the products was filtered on a Buchner funnel.
5. The product was washed with cold ethanol and was allowed to suck dry.
6. The yellowish product was recrystallized from ethyl acetate.
7. After recrystallization, a yellow crystalline was obtained.
8. The weight, yield, and melting point of the product were determined.
9. The UV and IR spectra of dibenzalacetone were ontained.
Results:
Weight of watch glass = 36.1291 g
Weight of watch glass + products = 45.6878 g
Weight of products = 9.5587
Melting point of products = 109 °C
Moles of benzaldehyde used = 0.1 mol
Moles of acetone used = 0.05 mol
Moles of dibenzalacetone produced = Moles of acetone used
= 0.05 mol
Theoretical weight of dibenzalacetone produced = 0.05 mol * 234.29 g mol-1
= 11.7145 g
Percentage yield of dibenzalacetone = 9.5587 g / 11.7145 g × 100 %
= 81.60 %
Discussion:
Condensation is a process which joins two or more molecules usually with the loss of a small molecule such as water or an alcohol. Aldol condensation (Claisen-Schmidt reaction) definitely is a process which join two carbonyl groups with a loss of water molecule in order to form β-hydroxyketone. The product is also known as adol because it containing two functional groups which includes aldehyde (or ketone) group and alcohol group. The product dibenzalacetone was formed from the reaction between an acetone molecule and two benzaldehyde molecules. Generally, the aldol condensation is carried out under a base condition.
Sodium hydroxide was mixed with distilled water then was used to react with sufficient ethanol as the first step. The particular reaction is an exothermic reaction which released the heat energy to the surrounding from the reaction. The sodium hydroxide was functioned as a catalyst in the reaction. The ethanol acts as a solvent which allows the acetone and benzaldehyde to dissolve and react with each other. After that, acetone and benzaldehyde were mixed in the solvent which turns to yellow colour quickly. Eventually, the product was formed with a yellow precipitate appear in the reaction after a few seconds. However, there are some impurities and side products were formed in the yellow precipitate. So, recrystallization was carried out by using ethyl acetate as solvent in order to purify the product and hence a pure product could be obtained for the ultraviolet (UV) and IR spectra analysis. In the recrystallization process, the yellow precipitate in ethyl acetate was immersed into an ice-bath in order to obtain a higher yield of product. This is because the heat energy in the precipitate easily to be released since the precipitation formation is an exothermic reaction and hence it maximizes the formation rate of the product.
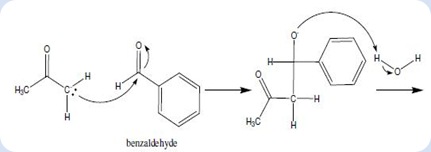
Acetone is considered as a stable and unreactive compound, so it should be converted into anionic form to increase its nucleophile properties to initiate the reaction. The sodium hydroxide dissolves in water to produce hydroxide ion and it tends to attack the α-hydrogen in acetone and to form water molecule. The deprotonation of acetone caused the enolate ion was produced as nucleophile which will be used in the synthesis of dibenzalacetone. An enolate ion was formed which it exists as resonance-stabilized structure which shown in the following diagram:
Diagram 1
The acetaldehyde enolate ion attack to the benzylic carbon of benzaldehyde via nucleophilic addition to form the intermediate as shown in below:
Diagram 2
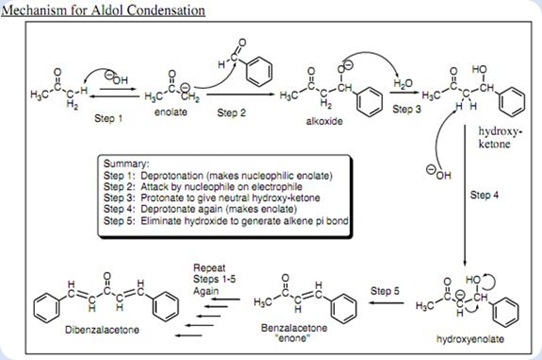
The oxygen attached to the benzylic position of carbon tends to attract one proton from water molecule to form hydroxide group in the intermediate. This is the formation of an aldol since the molecule consists of a carbonyl group and an alcohol group. In the basic condition, the hydroxide ion tends to remove one proton from the α-carbon resulting the formation of C=C double bond at the α and β carbon. At the same time, the hydroxide group attached to the β carbon forms a leaving group. After the condensation, benzalacetone was formed after two water molecules leaved as shown:
Diagram 3
The benzalacetone tends to form benzalacetone enolate ion after the hydroxide group from the surrounding attack the proton which attached to the carbon at benzylic position.
Diagram 4
The same process has been take place as in the Diagram 2 but with the more bulky benzalacetone enolate ion as the material. The benzalacetone enolate ion acts as a nucleophile which attacks another benzaldehyde. The protonation of the aldol took place followed by the hydroxide groups have been eliminated as leaving groups. As a result, the nucleophilic addition and base-catalyzed dehydration lead to the formation of the desired product which is dibenzalacetone. The mechanism of dibenzalacetone formation was shown in the Diagram 5:
Diagram 5
The overall mechanism of the dibenzalacetone was summarized in the Diagram 7 as shown in below:
Diagram 7
The percentage yield of dibenzalacetone in this experiment is 83.27%. Some of the product has been lost during the process of recrystallization. In recrystallization, some of the product dissolved in the ethyl acetate. The melting point of the product is lower than the actual melting point (110 °C ~ 111 °C). This is because there is some impurities exist in the particular compound which will tend to lower the melting point of the dibenzalacetone.
Precaution steps:
1. Avoid to carry out the experiment near the fire since the organic solvent are mostly flammable.
2. Avoid to smell benzaldehyde directly.
3. Handle carefully with sodium hydroxide since it is corrosive.

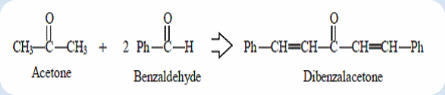




Glorious. Simply glorious. Well explained.
ReplyDeleteThis comment has been removed by the author.
ReplyDeleteVery straight forward...
ReplyDeleteI like the explanation given
Very Resourceful
ReplyDeleteVery useful..Thanks
ReplyDeleteThank you! Very helpful!
ReplyDeletegreat this is much easy to understand thanks a lot.
ReplyDeleteVery useful,simple & precise explanation.
ReplyDeleteNice explaination.
ReplyDeletethanks you. the artical is very useful
ReplyDeletedope
ReplyDeleteThis comment has been removed by the author.
ReplyDeleteIn step 5, why do we wash the product with cold ethanol?
ReplyDeletethanks a lot
ReplyDeleteI used the same procedure but ended up in an oily liquid. would like to know why can u help??
ReplyDeleteIncomplete removal of NaOH results in an oily product during recrystallization.
Deletewow well explained...can you write another article for malonic ester synthesis
ReplyDeletefantastic
ReplyDeletewow fantastic
ReplyDeleteWell explained
ReplyDeleteThank you , very clear explain , I hope the references mentioned , so we can used it in our reports
ReplyDeleteFull detailed and clear
ReplyDeletethanks a lot
ReplyDeleteOooo really bate
DeleteThnx
ReplyDeleteIt help a lot..
Really good
ReplyDeletewoow what a good explanation
ReplyDeletegreat explanation
ReplyDeletewow it's wonderful
ReplyDeleteNice and explicit explanations
ReplyDeleteI appreciate your work...very resourceful information
ReplyDeleteYellow color kyu aata hai sir??
DeleteBig up,,,, the work has been of great help on my side
ReplyDeletevery educative
ReplyDeleteexcellent job.
ReplyDeleteNice one
ReplyDeleteVery helpful,thanks
ReplyDeleteVery detailed and nice work.
ReplyDeleteIt's more reliable and easy to carry out
ReplyDeleteExplained in details and easy for understanding ,,,,big up
ReplyDeleteFully detailed...nice work
ReplyDeleteVery helpful. Which starting organic reagent should be in excess and why.
ReplyDeleteVery helpful. Which starting organic reagent should be in excess and why.
ReplyDeleteI have carried out same procedure 2-3 times but obtained brown coloured solution not yellow PPT. Why?
ReplyDeleteThanks a lot
ReplyDeleteVery help so much
감사합니다
Thanks a lot .....its very helpful ....all steps clear with very clearity
ReplyDeleteWhat is difference between Cliasen-Schmidt Reaction and aldol condensation?
ReplyDeletevery helpful, thank you
ReplyDeleteGot to know the uses of ethyl acetate, thanks for sharing. Get ethyl acetate at best price in India from Pon Pure Chemicals Group.
ReplyDeleteRGQ4R548Q36TR7GFVQI346TQ347QT347FV7YASERTQ34Q34TQE8F7YQWOQ3487TQ47TRQIEF6TVQ73RQT34YQSDFOVQ34Q3T8Q375TYDGYW45Q85TYQ[UUWEJFQP34RQ347Q3TRQ34TQ837YTVQAOVQ38Q43TV8Q34YTQ8PYE8UFDVTWQ45TQ4W5T8Q7YPAEJS
ReplyDeleteVery brilliant ideas
ReplyDeleteIt's a great explanation with total research appreciate it
ReplyDeleteYour content is most useful for us, Hunan Lianyi Industry & Trade Co., Ltd is the best chemical provider in china.
ReplyDeleteNaOH
Sodium hydrogen carbonate
Sodium carbonate
Natrium fluoride
NaHCO3
Feel free to contact us at any time.
Will it affect the experiment if I add aldehyde first?
ReplyDeleteWell explained and easy to understand
ReplyDeleteTop post. I look forward to reading more. Cheers american safety and health institute cpr test answers Wonderful great going, I love your work and look forward for more work from your side. I am a regular visitor of this site and by now have suggested many people.
ReplyDeleteVery helpful, thank you so much
ReplyDeleteThe explanation is on point
ReplyDeleteThanks so much
Thank you. I can now write my lab report.
ReplyDeleteVery helpful thank you
ReplyDeleteexcellent
ReplyDeleteWhat a wonderful explanation. excellent work
ReplyDelete