Objectives
1. To separate benzoic acid from impurities by recrystallization.
2. To learn the technique of recrystallization.
3. To determine the percent recovery of benzoic acid from recrystallization.
Introduction
A pure compound is a homogeneous sample that consisting only of molecules having the same structure. However, each substance believed to be pure may actually contain small amounts of contaminants. This includes the formation of side products during reaction, unreacted starting materials, inorganic materials, and solvents. However, recrystallization technique can be used to purify a solid and remove the impurities.
Recrystallization is a method of purifying a solid which takes the advantage of differences in the solubility of the desired products and impurities to obtain the pure desired products. Almost all solute are more soluble in hot solvent than in a cold solvent. Thus, if a solid is dissolved in a hot solvent but is insufficient to dissolve it in the cold solvent, the crystals should form when the hot solution is allowed to cool. In the simplest case all of the impurities present in a solid sample will be so much more insoluble in the chosen solvent that all that remains in solution is the pure dissolved product (the solute).
Step 1: Choosing the solvent
An essential characteristic of a successful solvent is that the compound be soluble in the hot solvent but insoluble when the solvent is cold. Tests can be performed with small amounts of material in test tubes: a few drops of a solvent are added and if the material proves insoluble then the tube is heated to see if the material will dissolve at a higher temperature-if so, then a good solvent for recrystallization of that material may have been identified. A solvent should be rejected if the material appears readily soluble in cold solvent, is not soluble to any appreciable extent in the hot solvent even when the volume of solvent is increased, or requires an impractically large volume in order to fully dissolve the crystals.
Step 2: Dissolving the sample
An Erlenmeyer flask should be used of such a size that it will only be filled to around half-way when all the solvent has been added. The solid sample is introduced together with around 75% of the amount of solvent thought to be required. It is always advisable to use less solvent at this stage. The flask is heated on a hotplate until dissolution of the solute is complete, additional solvent can be added to the hot solution as necessary to ensure complete dissolution. A boiling wooden stick should be added to provide a nucleation side for bubbles to form and facilitate an even boiling process. A process of gradual addition of solvent to the flask will ensure that the sample has dissolved to form saturea and will deposit crystalline material once it is cooled. Using excessive amount of solvents will only decrease the percent recovery of the products.
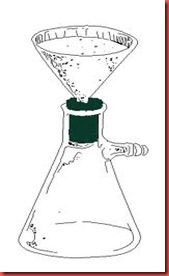
Step 3: Hot filtration
Once the solute is fully dissolved, the remaining impurities can be removed by filtering the hot solution through a filter paper folded into a cone and placed inside a glass filter. A problem here is that the solution will cool rapidly as soon as the Erlenmeyer flask is removed from the hotplate. In most cases, this problem can be minimized or avoided entirely by using a stemless funnel placed on the top of beaker containing a few millimeters of the recrystallization solvent. The beaker is placed on the hotplate and the boiling solvent serves to heat the funnel and prevent the solute from crystalling during the filtration process.
Step 4: Cooling
Cooling the filtered solution will allow crystals to form and rate of cooling can determine the size of the crystals formed. Fast cooling generally produced more crystals of relatively small dimensions, but slow cooling might allow larger crystals to form. The solution usually is left to cool to room temperature before cooled in the ice-bath to ensure maximum recovery.
Step 5: Cool filtration
When the crystallization process is judged to be completed the crystals need to be collected by suction filtration. Both the funnel and suction flask should be chosen so that neither will become more than half full during the filtration process. It is preferable that all of the crystalline material is being transferred to the funnel as a suspension in the crystallization solvent, however it is sometimes hard to get all of the crystals moving freely by swirling the flask and occasionally it will be necessary to add more ice-cold solvent in order to transfer the last of the crystalline material. It may also be necessary to dislodge crystalline material from the sides of the flask with a spatula prior to filtration.
Step 6: Washing the crystals
Once the suction filtration process is completed, the collected crystals should be washed with a little more ice-cold solvent to remove final soluble impurities which would otherwise be left on the surface of the crystals. The solvent used for this final washing should be as cold as possible to minimize losses from the crystals re-dissolving.
Step 7: Drying the crystals
Once the crystals have been collected on the suction funnel they can usually be satisfactorily dried by continuing to draw air over them for a few minutes. The almost dry crystals should then be spread on a filter paper to allow the last traces of volatiles solvent to evaporate.
Apparatus and Materials
Erlenmeyer flask (125 mL), short-stemmed funnel, hot plate, boiling chips, benzoic acid and charcoal.
Procedures
1. 2.0 g of crude benzoic acid were weighed into a 125-mL Erlenmeyer flask.
2. 200 mL of water was heated to boiling in a beaker on a hot plate with boiling chips.
3. An Erlenmeyer flask with a little water in it with boiling chips also was heated on a hot plate, with a short-stemmed funnel resting in its neck.
4. A filter paper was fluted to fit the funnel.
5. A few boiling chips was added to the benzoic acid and the adding of hot water to the benzoic acid was started until the benzoic acid has dissolved.
6. About 0.2 g of decolorizing charcoal was added.
7. The hot solution was filtered through the fluted filter paper into the heated flask.
8. The original flask and the filter paper were rinsed with a little hot water.
9. The solution of benzoic acid was removed from the hot plate and allowed to cool to the room temperature.
10. The solution is then cooled in an ice bath after 15 minutes for 10 minutes.
11. The crystals were collected by suction filtration using Buchner funnel.
12. Vacuum is continued to pull on the funnel for 5 minutes.
13. The filter paper with crystals was transferred onto a fresh piece of filter paper, and the crystals are allowed to air-dry.
14. The percent recovery and the melting point of benzoic acid were determined.
Results & Calculation
Weight of crude benzoic acid = 2.0007 g
Weight of filter paper = 0.8459 g
Weight of benzoic acid crystals + filter paper = 1.8776 g
Weight of benzoic acid crystals = 1.0317 g
Melting point of benzoic acid crystals = 120 °C
Percent Recovery
= weight of compound recovered / weight of compound started with x100%
= (1.0317 g / 2.0007 g) × 100%
= 51.57%
Relative Accuracy of Melting Point
= melting point of benzoic acid/ melting point of recovered benzoic acid × 100%
= (120 °C / 122 °C) × 100%
= 98.36%
Discussion
The percentage recovery of benzoic acid is only 51.57% may be due to several factors that caused the loss of products. One of the factors is that the volume of water added to the solution is too much which making the solution not saturated enough to produce maximum yield of benzoic acid after cooling. Normally, larger volume of water used will tend to the products to dissolve more easily. The benzoic acid crystallized on the filter paper during the hot filtration. The additional hot water need to be added to dissolve the benzoic acid crystals on the filter paper which causes the solution to be more dilute. So that, the products is lost in the solution.
Besides, too much decolorizing charcoal is added to the solution is considered as one of the factors. Decolorizing charcoal functions to provide vacant sites to the organic compounds to accommodate to it in which it removes the unwanted colored impurities. However, this also caused the loss of products in this process because some of the benzoic acid also will be adsorbed onto the surface of charcoal. Generally, the charcoal added should be only about 1-5% of the weight of the sample being recrystallized. A little amount of charcoal is sufficiently to remove the colored impurities. Otherwise, excessive use of charcoal will only caused the products to be removed together with the colored impurities.
In this experiment, the benzoic acid is dissolved in hot water while only a little amount of benzoic acid are able to dissolve in cold water. The benzoic acid cannot dissolve well in cold solution because of its hydrophobic benzene ring. However, the carboxyl group, -COOH that attached to the benzene ring allows some of the benzoic acid solubilise in water. In hot solution, the increase in temperature causes the water molecules has more kinetic energy and move faster. As a result, it allows the water molecules to penetrate through the benzoic acid solid and hence solubilization of benzoic acid occurs. In addition, the charcoal and other impurities present in mixture cannot dissolve in water. So, water is a good solvent to be chosen in the experiment.
The boiling chips were added in the experiment. Boiling chips are small, insoluble, and porous stones made of calcium carbonate or silicon carbide. There is a lot of pores inside the boiling chips in which it provides nucleation site to trap air and creates space to allow the bubble of solvent to form. When the boiling chips are heated, it will release tiny bubbles which can prevent bumping and boiling over of the mixture so that the loss of solution can be avoided even it is boiled. The adding of boiling chips must be added before boiling of solution instead of after boiling. This is because adding boiling chips to a solution near its boiling point will induce flash boiling as well. The boiling chips are not soluble in the solvent and hence they can be filtered out by using filter paper, but they are not reusable.
During the hot filtration, most of the charcoal powders were removed and stay on the filter paper while the benzoic acid solution pass through the filter paper and goes into the conical flask. However, some of the charcoal powder was noticed in the conical flask as well, although in a small amount. This might be due to the size of charcoal powder is too small that can pass through the pores of filter paper. During the cooling process, the hot solution was allowed to cool slowly to the room temperature, and then only immersed in an ice-bath. The solution should be protected from contaminants by covering with a piece of filter paper. Fast cooling always produces relatively small crystals because the particles do not have sufficient time to arrange themselves in proper conformation, so it is not advisable to cool down the hot solution immediately in ice-bath. The small size of crystal form may trap impurities easily. Oppositely, slow cooling allowed the molecules to interact and arrange themselves properly and hence they form larger size of crystals. But, large particles may causes some solvent being trapped inside the crystals.
During the cold filtration, the water soluble impurities that might dissolve in water which was filtered out through the suction filtration. However, some of the impurities might be trapped on the surface of the benzoic acid crystals, so a small volume of ice-cold water should be used to wash the benzoic acid crystals to dissolve the particular impurities. The crystal was dried in the oven at 100 °C. A fresh piece of filter paper can be used to place under the filter paper with benzoic acid crystals.
The purity of a crystal can be determined by its melting point. A narrow range of melting point indicates high purity of the sample, otherwise broad range of melting point indicates the presence of impurities in the crystal. The melting point of the recovered benzoic acid obtained experimentally is 120 °C. Compared to the pure benzoic acid with 122°C of melting point, the purity of the recovered benzoic acid is very high which is98.36%. Although the accuracy is high enough, but it also means that the compound is slightly contaminated with impurities which included the charcoal powder or the water molecules that trapped inside the benzoic acid crystals. The melting point of recovered benzoic acid is lower because the crystals cannot arrange properly due to impurities.
Structure of benzoic acid

An excellent article and well written !! Gives the in and out of a recrystallization !!
ReplyDeleteHumidifier Air Conditioner
Humidifier Air Cooler
ReplyDeleteHumidifier Air Cooler is a device which increases the humidity in air.
Thanks for sharing valuable post with us.
ReplyDeleteAir cooler
Air Cooler India
ReplyDeleteIts a valuable content shared,would like to know more about it.
Chemical Centrifuges
It very important sample to write other report thank you very much
ReplyDeleteThis is an great article, still modern to this time of year. It provides great information, which can be easily related to Science Assignments.
ReplyDelete🙏🙏🙏🙏🙏🙏🙏🙏🙏thanks a lot
ReplyDeleteHurrah, that's what I was play918kiss looking for, what a material! existing here at this web site, thanks admin of this web site.
ReplyDeleteThis is the best lab report ever ...thank you very much
ReplyDeleteI feel really happy to have seen your webpage and look forward to so many more entertaining times reading here. Same as your blog I found another one Sohman Epoxy
ReplyDelete. Actually, I was looking for the same information on the internet forSohman Epoxy
and came across your blog. I am impressed by the information that you have on this blog. Thanks once more for designs .
ReplyDeleteIam very happy to have such a blog here.I searched a lot and at last i find out the apt one..This blog is very informative for me and your knowledge about this topic is appreciable . Sohman Epoxy also contains a lot of information.This website also help me to deepen my knowledge..Just check this one also Xylene surely you may get some more details about the topic.Thank you.
ReplyDeleteHello! Someone in my Facebook group shared this website with us, so I came to give it a look. I’m enjoying the information. Wonderful blog and amazing design and style. iI know one website as you Sohman Epoxy .I really love the design of your website. Please check Sohman Epoxy .also similar to your blog. Thank you.