Objective:
1. To determine the type of food emulsion obtained by varying the mixture sequence
2. To produce the home made mayonnaise
Introduction:
Mayonnaise is a creamy, pale yellow, and mild-flavored food product which is frequently used in preparation of salads, sandwiches, and many other food products. Although the production of mayonnaise consisting of relatively few ingredients and processing steps, but successful formulation and processing are required an understanding of the role of each ingredient and the critical processing steps in order to create the delicate structure of mayonnaise. Mayonnaise is a unique emulsion which contains the mixture of water and oil. The major component in the mayonnaise is oil which dispersed throughout the lesser amount of continuous aqueous phase (water). The structure of mayonnaise is easily disrupted because of this unusual relationship between the oil and aqueous phase. Integration of processing and colloid chemistry is essential to understanding the formation and stabilization of the mayonnaise.
In this experiment, the mayonnaise is made by using 75% of oil, 8% of fresh egg yolk, 1% of mustard powder, 1.5% of salt, 1.3% of distilled water, and 13.2% of vinegar in producing mayonnaise. In a colloidal system, the minor component (dispersed phase) is usually being dispersed throughout the dispersion medium. However, the major component in mayonnaise (oil) is forced to be fine droplets to disperse throughout the lesser amount of continuous aqueous phase (dispersed medium). Mayonnaise is known as an oil-in-water (o/w) emulsion. When the emulsion of mayonnaise breaks, the water (dispersed phase) does not dispersed in oil (dispersed medium) and hence this allows the phenomenon of creaming formation to occur.
In order to maintain the stability of emulsion in mayonnaise, emulsifier acts as the most important role to stabilize the unique emulsion since the high amount of oil in water does not favor the formation of o/w emulsion. An emulsifier works at the surface of two immiscible liquids and tends to reduce to interfacial tension between two different liquids by reinforcing the in contact surface between them. The larger the ratio of surface area to volume of oil, the smaller the oil droplets dispersed in the water. Finer dispersion of oil droplets is required more emulsifier to surround them and thus this can maintain the stability of emulsion in the system. If emulsifier is not added, the two immiscible liquids will quickly separate after they mixed. Thus, emulsifiers are liaisons between the two liquids and serve to stabilize the mixture. In producing the mayonnaise, artificial emulsifier is not allowed. So, the source of emulsifier is normally obtained from egg yolk for stabilization of mayonnaise. The egg must be totally dissolved in the water before the addition of oil begins so that to achieve more efficient emulsion. Lecithin is a low molecular mass surfactant that can be found in egg yolk which acts as a hydrophilic effective emulsifier in oil-in-water emulsion.
Emulsion activity of emulsifier is based on its molecular structure. There is a hydrophobic part with a good non-aqueous solubility and hydrophilic part that is soluble in water. The hydrophobic part of the molecule is generally a long chain alkyl residue while the hydrophilic part of the molecule consists of a dissociable group. In a system containing two immiscible liquids such as oil and water, the emulsifier is located in the interface between two liquids which tends to reduce the interfacial tension of each liquid. The alkyl residues are solubilized in the oil droplets whereas the negatively charge of end group projected to the water. It maintains the stability of emulsion by involving the double electrostatic layer. The adsorption of negatively charge end group on the surface of oil globules causes the formation of a negative layer around them. Eventually, the oppositely charge particles will tend to approach to the particular negative layer and hence forming the second layer. This is known as double electrical layer. The electrical double layer creates repulsive forces greater than the attractive forces between the oil droplets. Hence, it can maintain the oil droplets from combining to each other in the emulsion.
Scattering light is one of the characteristics of colloidal system. Emulsion always exhibits the similar characteristic with colloidal suspension which is scattering the light. The oil-in-water emulsion present as a cloudy and turbid because of the existence of oil droplets dispersed in the aqueous phase. This is because the many phase interfaces between the two liquids scatter the light which passes through the emulsion system. The basic colour of emulsion is white. The Tyndall effect will scatter the light and distort the colour to blue if the emulsion is dilute. In a concentrated emulsion, the colour of emulsion system will scatter light and then distort the colour to yellow.
Several ways have been proposed to determine type of emulsion formed. The drop-dilution method can be used to determine the type of emulsion. To a small portion of the emulsion, add some water and stir slightly. If the water blends with the emulsion, it is an oil-in-water emulsion, but if oil blends with the outside phase it is a water-in-oil emulsion.
Another method of determining the type of emulsion is to use Sudan III, red dyes soluble in the oil but not in the water. A small portion of the finely powdered dye is dusted over the surface of the emulsion. If oil is the external phase the color gradually spreads throughout the emulsion. But if water is the external phase the color does not spread but is confined to the oil with which it comes in contact on the surface.
The microscope may be used to determine the type of emulsion formed. If the oil is dyed red, a red field with clear globules indicates a water-in-oil emulsion; red globules in a clear field show an oil-in-water emulsion. Sometimes a multiple emulsion is obtained, for example, a dispersed phase dispersed within a dispersed phase. The only means of identifying a multiple emulsion is by using the microscope.
Apparatus: beaker, measuring cylinder, glass rod
Materials: oil, egg yolk, salt, mustard powder, distilled water, vinegar
Procedure:
| Ingredients | % | Weight or volume |
| Oil | 75.0 | 75 g |
| Egg yolk | 8.0 | 8 g |
| Salt | 1.5 | 1.5 g |
| Mustard powder | 0.1 | 0.1 g |
| Distilled water | 1.3 | 1.3 ml |
| Vinegar, distilled 5% acetic acid | 13.2 | 13.2 ml |
| Total | 100.0 | 100 g |
In this formula, 30% of the aqueous ingredients equals to 99g water. In all the cases, make a paste of the mustard in a little water before using.
Procedure A
All the ingredients were mixed well in a beaker and stirred by using glass rod for 30 minutes.
Procedure B
1. The mustard powder, salt, water, vinegar, and egg yolk were mixed in a beaker.
2. The oil was slowly added into the mixture while stirring. Stir for 30 minutes.
Procedure C
1. Egg yolk was added to the beaker and blended thoroughly.
2. In a separate beaker, the mustard powder, 1.3g of water, 3.2g of vinegar, and salt were blended.
3. The mixture was stirred until all the salt dissolved.
4. The mixture was added into the egg yolk and was stirred for 5 minutes.
5. At this point, the oil was slowly added while stirring.
a. First 5 minutes, 10-15% of oil was added. The first addition should be small and gradual. Wait about 1 minute between additions.
b. During the next 15 minutes, 50% of oil was added.
c. During last 5 minutes, the remaining of oil was added.
6. Gradually add the remaining vinegar and stir for 5 minutes.
Take the observation on the three different mixtures on viscosity and dilution test.
| Mixture | Procedure A | Procedure B | Procedure C |
| Viscosity | Low viscous | Moderately viscous | Highly viscous |
| Dilution test | Water in oil (W/O) | Water in oil (W/O) | Oil in water (O/W) |



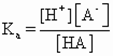
![clip_image009[4] clip_image009[4]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEgeD-WRJlESBMbl46xItvyFyk9vq7Dd0X2TGQzRhwPqnK-ke4iVKWRePfjYXfhyphenhyphenOIfHjk6qCur_sBhqY-JJd-w4cP1dZWT6O6xmGgobhTmFLuZ_tHQbocsC8hFG87-mDtrD1f8-bRwmSWQ0/?imgmax=800)