Objective:
1. To study and understand the formation of polyamide (nylon) through condensation polymerization between surface technique
2. To observe the effect of external factors on molecular weight and polymer yield
Introduction:
Polymerization is a process of forming long and repeating organic polymer chains. Polymerization is categorized into different systems based on the mechanism and structure of polymer. Both structure and mechanism are usually required in order to clearly classify a polymer. Polymers were originally classified by Carothers (1992) into addition and condensation polymerization. However, the more recent terminology further classifies polymerization into step-growth, chain-growth and ring opening polymerization.
Condensation polymers are refers to the polymers that were formed from polyfunctional monomers by various condensation reactions with the elimination of some small molecules such as water. The type of end product resulting from the condensation polymerization is depends on the type of functional end group which can react. For example, the formation of polyester between a dicarboxylic acid and a diol in which two monomers are linked together and a water molecule has been produced.
This process can continue adding alternatively one molecule and then the other, building the polymeric chain of hydrocarbon units that joined by ester bond. This is molecule formed from the condensation polymerization is called polyester.
In order for a condensation polymerization to occur continuously, the small molecules need to be removed from the system either by heating or through vacuum. However, heating would cause degradation or breaking of polymer molecules from bigger size of polymers to smaller size of polymer molecules. As a result, this will cause the formation of low molecular weight polymer. In order to overcome this problem, polymerization should be carried out at low temperature either by using the method of polymerization in solvent or polymerization between two surfaces of solution (interfacial polymerization). Many polymers can be produced at lower temperature (instead of high temperature) by using Schotten-Baumann reaction of acid chlorides.
Interfacial polymerization is refers to the condensation polymerization between two reactants is carried out at the interface of two immiscible solvents in which each containing one of the reactants. Interfacial polymerization is important because it produces the polymer with high molecular weight in a short period of time which under normal conditions of temperature and pressure. This type of polymerization could be carried out at room temperature and the types of polymers that could be formed using this technique are polyurethane, polyamide and polyurea. Thus, the formation of nylon 6,10 which is a type of polyamide that can be produced by using this method of reaction between two surfaces of immiscible solvents. This technique is extremely useful to the reactants which are not stable under the polymerization conditions by other techniques. Among the techniques of condensation polymerization, interfacial polymerization has some added advantages over the other techniques.
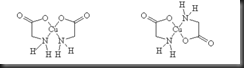
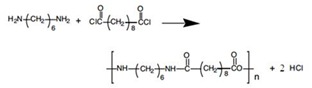
Nylon 6,10 is produced from the two monomers named sebacoyl chloride (ClOC(CH2)8COCl) and 1,6-diaminohexane (also known as hexamethylenediamine, H2N(CH2)NH2). Sebacoyl chloride is a 10-carbon acid chloride with a –COCl group as functional group at each end. The other monomer is a 6-carbon chain with two amino groups, -NH2 at each end. Nylon 6,10 is named based on the carbon number of two monomers, in which the first number come from amine monomer while the latter one from acid chloride monomer. These two compounds are polymerized via condensation with the loss of a molecule of hydrogen chloride, as shown in the equation below:
Nylon is a term to represent synthetic polyamides. Different nylons are described by a numbering system that shows the number of carbon atoms in the monomer chains. Nylons from diamines and dicarboxylic acids are designated by two numbers in which the first representing the diamine whereas the second representing the dicarboxylic acid. In order to generate a long chain polymer and to expedite the rate of kinetic reaction, the polymerization system should be stirred vigorously to make sure that a new surface of polymer is always produced in the system. This ensures that the polymers produced would give a high percentage of yields. Both hexamethylenediamine and sebacoyl chloride are not solvents in the polymerization process between the interface of solutions. In the stirring technique, the non equilibrium system that occurs eliminates the weakness that might occur in polycondensation process such as:
1. The necessity of high temperature
2. Longer time of reaction
3. Accurate stoichiometric balance of equivalent
Physical properties of nylon 6,10:
1. Bright yellow in colour
2. Less transparent
3. Melting point of 220 °C
4. Moderate crystallinity
5. High impact strength
6. Electrical insulating ability
Apparatus:
100ml beaker, 10ml graduated cylinder, glass rod, thin stick
Materials:
60% w/v hexamethylenediamine, sebacoyl chloride, sodium laurel sulphate (soap powder)
Procedures:
1. 3ml sebacoyl chloride solution and 100ml of hexane (solution B) were added into a 100ml beaker.
2. 0.01g of soap powder was added into the mixture (solution A) of 3ml of hexamethylenediamine, 50ml of water and 2 drops of phenolphthalein.
3. Solution A was added carefully into solution B so as not to disturb the interface between two solutions.
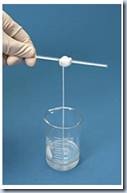
4. A layer of polymer was formed between solution A and solution B. The strand of polymer (nylon) was wrapped around the glass rod and nylon strand was winded onto the rod with a steady pace.
5. The polymer formed was rinsed with water.
6. Let it dry in the oven.
7. The weight of polymer was measured.
8. The steps above were repeated with 5ml of solution B and 3ml of solution A. The solutions were stirred.
9. The polymers formed were characterized and compared.
Results and calculations:
Table 1 Observation on polymers that made with different methods
| Observations on polymers formed with different methods | |
| Wrapping on glass rod | White, long and weak elasticity nylon was formed. |
| Stirring | A clump of nylon was formed. |
Table 2 Weight of polymer obtained by wrapping on glass rod
| Weight of empty watch glass | 20.4850 g |
| Weight of (empty watch glass + polymer, Nylon 6,10) | 20.9122 g |
| Weight of polymer (nylon 6,10) | 0.4272 g |
Table 3 Weight of polymer obtained by stirring
| Weight of filter paper | 0.4836 g |
| Weight of (filter paper + polymer, Nylon 6,10) | 0.9099 g |
| Weight of polymer (nylon 6,10) | 0.4263 g |
Calculate percentage yield
Unstirred interfacial polymerization
| Types of monomer | hexamethylenediamine | Sebacoyl chloride |
| Density (g cm-3) | 0.84 | 1.121 |
| Volume used (cm3) | 3.00 | 3.00 |
| Molecular weight (g mol-1) | 116.00 | 239.00 |
Mass of hexamethylenediamine = 0.84 g/cm3 x 3cm3 x 60% = 1.512g
Theoretical number of mole of hexamethylenediamine = 1.512g / 116 g mol-1
= 0.013 mol
Mass of sebacoyl chloride = 1.121 g/cm3 x 3cm3 = 3.363g
Theoretical number of mole of sebacoyl chloride = 3.363g / 239 g mol-1
= 0.014 mol
Thus, limiting agent is hexamethylenediamine.
Molecular weight of repeating unit = 282 g mol-1
Weight of nylon 6,10 obtained experimentally = 0.4272g
Actual number of mole of repeating unit = 0.4272g / 282 g mol-1 = 0.0015 mol
Percentage yield = 0.0015 mol / 0.013 mol x 100%
= 11.54%
Stirred interfacial polymerization
| Types of monomer | hexamethylenediamine | Sebacoyl chloride |
| Density (g cm-3) | 0.84 | 1.121 |
| Volume used (cm3) | 3.00 | 5.00 |
| Molecular weight (g mol-1) | 116.00 | 239.00 |
Mass of hexamethylenediamine = 0.84 g/cm3 x 3cm3 x 60% = 1.512g
Theoretical number of mole of hexamethylenediamine = 1.512g / 116 g mol-1
= 0.013 mol
Mass of sebacoyl chloride = 1.121 g/cm3 x 5cm3 = 5.605g
Theoretical number of mole of sebacoyl chloride = 5.605g / 239 g mol-1
= 0.023 mol
Thus, limiting agent is hexamethylenediamine.
Molecular weight of repeating unit = 282 g mol-1
Weight of nylon 6,10 obtained experimentally = 0.4263g
Actual number of mole of repeating unit = 0.4272g / 282 g mol-1 = 0.0015 mol
Percentage yield = 0.0015 mol / 0.013 mol x 100%
= 11.54%
Table 3 Comparisons between two polymers that produced by different methods
| Wrapping on the glass rod | Method to produce polymers | Stirring by using glass rod |
| 0.4272g | Mass | 0.4263g |
| 11.54% | Percentage yield | 11.54% |
| More elastic | Elasticity | Less elastic |
| Stronger | Stiffness (Malleability) | Weaker |
| Stronger | Ability to stick to surface | Weaker |
Discussion:
In this experiment, the interfacial polymerization technique plays a very important role. This method is primarily used in the case of condensation polymers, in which two reactants have different solubility characteristics. Hexamethylenediamine and sebacoyl chloride were used in this experiment in order to demonstrate the condensation polymerization in the interface between two solutions.
Interfacial polymerization is different from the usual condensation polymerization. This is because the monomers (undergo this polymerization) tend to diffuse to the interface and will only react with the polymer chain end formed initially. The diacid chloride and diamine monomer molecules will react with the growing polymer chain end before they penetrate through the polymer film to create the growth of new polymer chains. Therefore, interfacial polymerization creates a high tendency to generate high molecular weight polymer in the polymerization process. In addition, stoichiometry automatically exists at the interface because both monomers diffuse from aqueous phase and organic phase respectively. The stoichiometry at the interface is perfectly controlled by mass transfer controlled rate of diffusion and consumption of each monomer species.
Sebacoyl chloride was placed in 100ml of hexane (solution B) while hexamethylenediamine was added with 50ml of water (solution A). The introduction of aqueous layer and organic layer was used to create an interface between two solutions since aqueous layer and organic layer will not mix together. Interfacial polymerization occurs instantly forming a thin film of solid nylon 6,10 at the interface. The thin film at the interface stops further reaction by preventing the monomers to meet each other. During the preparation of solution B, any apparatus used should be rinsed with hexane and thus they do not contain water. If water present, chloride ion, Cl- of sebacoyl chloride tend to react with the hydroxide, OH- in water to form sebacic acid as well as two hydrochloric acid molecules.
The reaction between sebacoyl chloride and water is very reactive since chloride ion is a group leaving group.
A small amount (0.01g) of sodium laurel sulphate (soap powder) was introduced into the solution B in order to lower the surface tension of solution A and hence hexamethylenediamine will easily solubilize in it. As a result, the condensation polymerization is encouraged to occur in the interface. Since hexamethylenediamine exists its base property in the presence of water, so two drops of phenolphthalein was added as an indicator to show the position of interface between the solutions and to determine the point where hexamethylenediamine has been used up. In alkaline solution, the colour of phenolphthalein is pink while it will be faded if the alkalinity of solution decreases.
The interface between solution A and solution B should not be disturbed during transferring the solution A into solution B. This is to make sure both reactants do not form a polymer clump in unstirred interfacial polymerization. The system contains two layers in which solution A with hexamethylenediamine is upper layer whereas solution B with sebacoyl chloride is the lower layer because sebacoyl chloride is denser than hexamethylenediamine. During the transferring process, condensation polymerization between hexamethylenediamine and sebacoyl chloride took place at the interface is shown as the equation below:
At the interface between the solutions, a bigger molecule was formed from the reaction between both monomers and two small molecules hydrochloric acid, HCl were generated. The bigger size molecule formed at the interface could be dimer, trimer, tetramer, oligomer and polymer.
In unstirred interfacial polymerization, the colour of phenolphthalein indicator was faded as more nylon was wrapped on the glass rod and finally it was decolourized. This is because the decrease in concentration of hexamethylenediamine in which the diamine was polymerized in the system. In addition, the condensation polymerization produced hydrochloric acid as by-product and it neutralized the acid present in the system. The increase of hydrochloric acid concentration caused the phenolphthalein indicator to be decolourized. In stirred interfacial polymerization, the pink colour of phenolphthalein decolourized immediately once both solutions were stirred. This method produced a polymer clump in the solution. As the time pass, the polymer clump was predicted to become smaller because the hydrochloric acid will hydrolyze the amide bond in the polymer molecule.
By comparing both polymers that produced from two methods, the nylon 6,10 in unstirred polymerization results a more elastic and stiffer polymer than the nylon 6,10 in stirred polymerization. Besides, its ability to stick on the wall of beaker and surface is stronger when compared to nylon obtained from the latter method. The molecular weight of nylon obtained in unstirred polymerization is predicted to have higher molecular weight since its mechanical strength is greater than the other one. The weight of nylon 6,10 obtained in the unstirred interfacial polymerization was 0.4272 g while the weight of nylon 6,10 obtained via stirred interfacial polymerization was 0.4263g. Both methods gave the same percentage yield of 11.54%.
Reference:
1. Odian, G. (2004). Principles of Polymerization (4th Ed.). USA: John Wiley & Sons, Inc.
2. Wagh, S.J., & Jadhav K.T. (n.d.) A review on interfacial polycondensation:
types, applications andeffect of various parameters.
3. Ji, J., Childs, R.F., & Mehtra, M. (2001) Mathematical model for encapsulation by interfacial polymerization. 192 (2001), P 55-70.









![clip_image002[4] clip_image002[4]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEgKsNlsE2bhil-D_Hh_go-LlAYu1UoDFiYRDDpHHGW4M56m9tNtvu5jXyC_CyqfaTS_K4M7sfXR8U9S9I8RlV3e-BdlPofdOzZ1vML6CicGgM1AtGC-xMoy8T2OpvfLPAEOHb5_AwLNINV-/?imgmax=800)
![clip_image004[4] clip_image004[4]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEhUFC-iT0jnCBKPjMMX5Uw1laiVfaldQ1FRkOQUcF7YUZww7biZ-05WREIC69xE7O-7o_SpRjLtqUw_w43-7mdsMkTXDwzHDMKXNoOqvmzadxSQ_G8qL6UQqp0hl-7UiOX667G4a6QOIpW-/?imgmax=800)