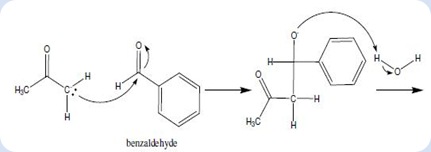
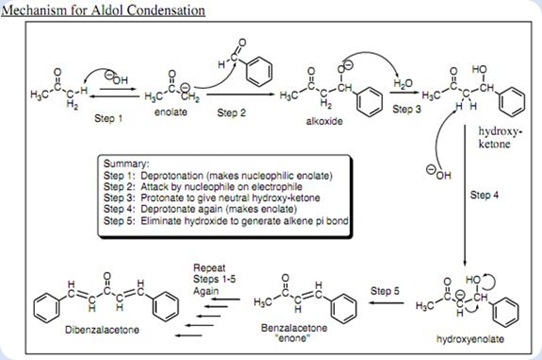
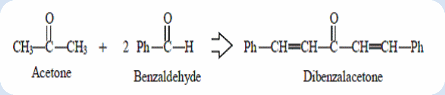Objective:
1. To synthesis triphenylmethanol from Grignard reaction
2. To study the method to produce Grignard reagent
Introduction:
Grignard reagents are organomagnesium halides (RMgX), and are one of the most synthetically useful and versatile classes of reagents available to the organic chemist. An alkyl, benzyl, or aromatic halide is reacted with a magnesium metal by using an anhydrous solvent in order to produce Grignard reagent. Ether or tetrahydrofuran are usually can be used as the anhydrous solvent in producing the particular reagent. Tetrahydrofuran is a strong base and it has a better solvating ability, it may used when Grignard reagent does not readily form in diethyl ether. This is considered as an organometallic compound which consists of the combination of a metal and organic molecule. Figure 1 in below shows the general reaction mechanism for the formation of Grignard reagent.
The chemical reaction between an organic halide and a magnesium metal can produce an alkyl or aryl free radical and magnesium free radical. The formation of Grignard reagent has been occurs. The bonding between carbon and magnesium is a covalent bond but it is highly polarized because the magnesium is bonded to halide which is an electron withdrawing group. This causes the formation of partial positively charge and partial negatively charge on the magnesium atom and alkyl or aryl group respectively. Hence, the carbanion has both characteristics of a good nucleophile and a strong base. Its basicity allows it to react with the electrophile carbon in a carbonyl group. Besides, Grignard reagent also works with acidic compound such as carboxylic acid, phenol, thiol, alcohol, and even water. One of the most important reactions is the addition of Grignard reagent to the carbonyl compound like aldehyde, ketone, and ester in order to produce the corresponding secondary alcohol and tertiary alcohol.
Figure 2
The most challenging part of this experiment is to avoid the Grignard reagent contact with water. The partial negative charge on the carbon atom that bonded to magnesium exhibits a very basic property. The water molecule will destroy the nucleophilic property of Grignard reagent. So, several precaution steps must be taken in the procedures to avoid the Grignard reagent reacts with water: the reaction flask is dried in the oven before use; iodine is vaporized in the flask tie up traces of water and to activate the surface of magnesium; the anhydrous diethyl ether should be used.
Figure 3
The figure 3 above shows the Grignard reagent and water reaction. The metal hydroxide (alkoxide) formed in the above reaction is appears as insoluble white solid (HO-MgBr or RO-MgBr) in the diethyl ether solvent. Thus, the process of producing Grignard reagent must be start over again in a dry glass if the insoluble white solid is observable during the formation of Grignard reagent.
For a variety of reasons, anhydrous diethyl ether is the solvent choice for carrying out a Grignard synthesis. One of the reasons is the vapors of the highly volatile diethyl ether helps to prevent the oxygen in the atmosphere to reach the reaction solution. Grignard reagent will reacts with oxygen which hydroperoxides is produced. This compound is highly unstable if exposed to the air so that the compound is usually not isolated from the solvent. Other than that, the basic oxygen atoms in ether molecules are actually coordinate with and help to stabilize the Grignard reagent. The figure 4 below shows how the anhydrous diethyl ether protects Grignard reagent from oxidation:
Figure 4
There is a layer of oxide coated on the surface of magnesium which used to synthesis Grignard reagent. The oxide layer works to prevent it to react with alkyl bromide. The formation of Grignard reagent is highly exothermic which will produce a lot of heat energy from the system. Once the reaction has been initiated, the system will reflux itself in the bottom flask without any external heat source. The adding of a drying tube that contains calcium chloride to the reflux apparatus is used to protect the reaction from atmospheric moisture.
In this experiment, bromobenzene is the alkyl halide used to generate Grignard reagent.
Figure 5
Once the Grignard reagent is readily formed, the carbonyl compound has been introduced into the reagent in order to synthesis the expected product. The methyl benzoate (ester) acts as the carbonyl containing compound in the experiment. The reaction between methyl benzoate and Grignard reagent is showing in the following figure 6:
Figure 6
Dissociation of magnesium alkoxide produces a ketone which tends to react further with more Grignard reagent. The final step of the synthesis is involving hydrolysis of the magnesium alkoxide by using a mineral acid. As the result, the reaction synthesizes an alcohol, triphenylmethanol and magnesium salt (water soluble).
A side reaction may take place in the reaction between phenylmagnesium bromide and bromobenzene. The side product has been produced is biphenyl which consists of two phenyl rings. The biphenyl is known as impurity in the experiment. The impurity can be removed from the product through a method of recrystallization since biphenyl is much more soluble in ligroin compared to triphenylmethanol.
Apparatus: dropping funnel, two neck round bottomed flask, drying tube, condenser, sonicator, magnetic stirrer, hot plate, separating funnel, beaker, Buchner funnel, glass funnel, melting point apparatus
Materials: magnesium turning, anhydrous diethyl ether, bromobenzene, calcium chloride, methyl benzoate, iodine crystal, 10% H2SO4, ice, sodium bisulfate, sodium sulfate anhydrous, petroleum ether, methyl spirit
Procedure:
Part A – Preparation of phenylmagnesium bromide (phenyl Grignard reagent)
1. A condenser and a 50ml dropping funnel were set up to a 250ml two neck round bottom flask.
2. A calcium chloride drying tube was inserted into the top of the condenser.
3. 1.4g of magnesium turning was weighed and placed into the two neck round bottom flask with a stir bar. 10ml of anhydrous diethyl ether was added immediately into the two neck bottom flask.
4. 6.2ml of bromobenzene and 30ml of anhydrous diethyl ether were added into the 50ml dropping funnel.
5. 5ml of the mixture in dropping funnel was added to the ether/magnesium mixture. The mixture is stirred with a magnetic stirrer.
6. If the reaction does not begin immediately, both palms were placed around the bottom of the flask to keep it warm.
7. A small amount of iodine crystal was added directly into the magnesium surface if the reaction does not take place after 5-10minutes.
8. Once the reaction was initiated and the formation of Grignard reagent became steady, the ether refluxed itself. The remaining mixture in dropping funnel was added dropwise into the round bottom flask.
9. The solution was allowed to reflux for 10 minutes.
Part B – Preparation of triphenylmethanol from Grignard reagent
1. After reflux, the round bottom flask was cooled down in ice bath and a solution of 3.2 ml of benzoate dissolved in 15ml anhydrous diethyl ether was placed in the dropping funnel.
2. The solution was added over 5 minutes to avoid the solution to get too exothermic.
3. Once the reaction was completed, the solution was heated to reflux for 10 minutes to complete the reaction.
4. After reflux, the round bottom flask was cooled with ice bath and the mixture was poured into a 600ml beaker with contains 75g of ice and 30ml of 10% H2SO4.
5. The mixture was stirred until all white solid was dissolved.
6. The mixture was poured into the separating funnel and separated the two layers. The ether layer was washed with 15ml of H2SO4, followed by 15ml of water and then with a solution of 1g of sodium bisulfite dissolved in 12ml of water.
7. The ether layer was dried over sodium sulfate anhydrous and filtered with cotton wool. 15ml of petroleum ether was added to the ether layer. The ether layer was concentrated over a steam bath at 60-80 °C until the triphenylmethanol was formed. The product was cooled in an ice bath.
8. The product was recrystallized from methyl spirit.
9. The weight, yield and melting point of triphenylmethanol were determined.
Results and calculation
Weight of magnesium = 1.4009g
Volume of bromobenzene = 6.2ml
Volume of methyl benzoate = 3.2ml
Weight of watch glass = 14.8091g
Weight of watch glass + weight of product = 13.7410
Weight of product = 1.0681g
Atomic weight of magnesium = 24.31g/mol
Number of mole of magnesium = 1.4009 g / 24.31 g mol-1
= 0.0576mol
Molecular weight of bromobenzene = 157g/mol
Density of bromobenzene = 1.495 g/cm3
Weight of bromobenzene = density x volume
= 1.495 g/cm3 x 6.2cm3
= 9.269g
Number of mole of bromobenzene = 9.269g/ 157 g mol-1
= 0.0590mol
Since the magnesium is limiting reagent, so the number of mole of Grignard reagent produced is limited by number of mole of magnesium.
Number of mole of Grignard reagent produced = 0.0576mol
Density of methyl benzoate = 1.0837 g/cm3
Weight of methyl benzoate = density x volume
= 1.0837 g/cm3 x 3.2cm3
= 3.468 g
Molecular weight of methyl benzoate = 136.144g/mol
Number of mole of methyl benzoate = 3.468 g / 136.144g mol-1
= 0.0255 mol
Since the Grignard reagent is excess, the product is limited by methyl benzoate so that it is limiting reagent in the reaction.
Thus, the number of mole of triphenylmethanol = 0.0255 mol.
Molecular weight of triphenylmethanol = 260.318g/mol
Theoretical weight of triphenylmethanol = 0.0255mol x 260.318g/mol
= 6.6381 g
Actual weight of triphenylmethanol =
Percentage yield = actual yield / theoretical yield x 100%
= 1.0681g / 6.6381g x 100%
= 16.09%
Melting point of triphenylmethanol = 153°C ~ 156°C
Discussion:
The purpose of this experiment is to synthesis triphenylmethanol by using Grignard reagent. In order to synthesis triphenylmethanol, Grignard reagent is playing an important role because Grignard reagent is the key reagent in this experiment. The presence of water in the process of generating Grignard reagent will causes the particular reagent to be decomposed. So, the solvent used in the experiment must not contain any water such as diethyl ether since it is a water free solvent. In order to make sure the water in air can be eliminated, a small amount of calcium chloride has been placed with the drying tube on the top the condenser. The calcium chloride acts as a drying agent which to absorb all the water from the air in the reflux apparatus and it prevent the atmospheric moisture. The purpose of using magnetic bar is to increase the rate of reaction for Grignard reagent.
In order to produce Grignard reagent, the magnesium turning was added with anhydrous diethyl ether. Magnesium turning (thin shaving with high surface area) is usually used in preparation of Grignard reagent due to its large surface area that can increase the reaction rate. The diethyl ether functions as the medium for the Grignard reaction to take place and stabilize the reagent. This is because the solvent (diethyl ether) is highly volatile solvent which can prevent the water from atmosphere approaching to the Grignard reagent after the Grignard reagent is formed. Besides, diethyl ether is easily removed from the reaction mixture since it has a low boiling point of 36°C. A mixture of bromobenzene and diethyl ether was prepared in the dropping funnel. The adding of diethyl ether in the mixture is works for the similar function which make sure the solvent is free from water. The addition of ether and bromobenzene mixture into the magnesium surface might not allow the reaction to occur due to lack of heat energy.
The iodine crystal was added into the magnesium surface because the heats from water bath or palm were not enough to initiate the reaction. Alternatively, the iodine crystal was added instead of increasing the temperature that supplied to the system in order to prevent the explosion since diethyl ether is highly flammable. The iodine crystal facilitate the reaction either activating the magnesium through removal of its oxide coating or by oxidizing the bromide in organic compound to form negatively charged bromide which is more reactive towards magnesium. A second alternative is place the flask containing reaction mixture over a sonicator to start the Grignard reaction. The sonicator is used to produce ultrasonic wave in which helps to remove the oxide coating physically.
When the reaction has been initiated, the appearance of bubbles on the solvent surface indicated that the formation of phenylmagnesium bromide start to occurr. The bromobenzene is reacted with magnesium metal to form phenylmagnesium bromide which is known as Grignard reagent. The chemical reaction between magnesium and bromobenzene is shows in below:
The formation of Grignard reagent is solvated by diethyl ether which protects the reagent from attacked by water molecules. If the water reacts with Grignard reagent, the decomposition of the particular reagent will occur. The mixture of ether and bromobenzene was added slowly to make sure that the Grignard reagent form steadily in the reaction. The side product may be generated in high yield if the mixture is added in a large volume at the same time. The formation of Grignard reagent is an exothermic process. Thus, the system can refluxed itself without any heat supply to it.
After reflux, the Grignard reagent produced was cooled down in an ice bath in order to reduce its temperature. This is to prevent the immediate addition of solvent from evaporating quickly due to high temperature of Grignard reagent if cooling down process is not taken. The methyl benzoate is the subsequent reactant which was used to react with Grignard reagent in this experiment. In order to avoid the reaction between Grignard reagent and methyl benzoate get too exothermic, the methyl benzoate in anhydrous diethyl ether must be added in a small amount. The system was refluxed itself to produce (Ph)3CO-Mg-Br as product.
The Grignard reagent can be dissociated to form negatively charged carbanion which attacked the carbonyl carbon with partial positively charged. The carbonyl carbon of methyl benzoate was attacked by the nucleophilic carbanion during reflux. The nucleophilic addition of Grignard reagent to methyl benzoate caused the methoxide became the leaving group from the intermediate and the formation of benzophenone. Since the benzophenone consists of a carbonyl carbon as functional group, this favored the second nucleophilic attack of Grignard reagent and (Ph)3CO- anion with three benzene ring has been produced in the solution through reflux. The solution was treated by using sulphuric acid to protonate the (Ph)3CO- anion to generate the triphenylmethanol, (Ph)3COH as product. Triphenylmethanol has a synonym which is known as triphenylcarbinol. The formation of triphenylmethanol is highly exothermic, so ice bath was used to reduce the temperature and heat energy produced from the system. In this stage, some white solid were precipitated out in the cold solution, the white solid is the desired product. The mechanism of formation of triphenylmethanol by using Grignard reagent via nucleophilic addition is shown in the following Diagram 1:
Diagram 1
In order to remove the impurities and side product, the washing process is necessary. The ether layer was washed with sulphuric acid and followed by water. The aqueous solution was used to remove the water-soluble impurities in the mixture. Then, sodium bisulfide was a base which was used to neutralize the acid added before. Sodium sulfate anhydrous has been introduced to remove all the water in the mixture since it is a drying agent and the clump of solid sodium sulfate was filtered. In the process of producing triphenylmethanol, some side products have been produced at the same time such as biphenyl.
Therefore, petroleum ether was used in the experiment in order to let the biphenyl dissolved in it so that this side product can be removed via recrystallization. This might be due to the higher polarity of the triphenylmethanol compared to biphenyl which enables the triphenylmethanol to dissolve easily in the polar methyl spirit.
Recrystallization of triphenylmethanol has been carried out to purify the product. Methyl spirit (denatured alcohol) is a mixture of methanol and ethanol which was used as the dissolve medium in recrystallization. After recrystallization, the product is still not pure enough since its melting point of 153°C ~ 156°C is lower than the theoretical melting point which is 162°C. This deviation may be due to the product is not completely dry so that it affects the melting point of the product. The yield of the product in the experiment is 1.0681g which contributes to the percentage yield of 16.09%. The percentage yield is very low may be due to there are many impurities were formed in the reaction since the impurities compete the material which required for the formation of desired product.
Precaution steps:
1. Avoid the diethyl ether from any heat source since it is extremely flammable.
2. Carry out the reaction away from any heat source.
3. Place all the glassware in a 110°C in order to make sure all the glassware is totally dry.
4. Use solvent to wash the glassware instead of distilled water.







![clip_image010[1] clip_image010[1]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEhNF2C3KRqyZN9rj1gjHVtSoPqnVFtF4hhTVaHvJKuA4mt86wDsPlwpfCSGqtsZoMpGIp1zRh8PrwxTHig-UYUq-GApXz33RqefQ05LG4i9snWDLUoICheJmYBAJ_naVXzLTig-TJzj4Swg/?imgmax=800)