Objectives:
1. To produce tert-butyl chloride from tert-butyl alcohol
2. To understand the SN1 and SN2 mechanism involved in the reaction
3. To determine the yield of percentage of t-butyl chloride
Introduction:
Alkyl halide is also known as haloalkane or halogenalkane. Alkyl halide is a hydrocarbon group which attached with at least one halide atom in the molecule. Alkyl halides always resemble the parent alkanes in being colourless, relatively odorless and hydrophopic. Their boiling point is always increase as the parent chain increase, the longer the parent chain, the higher the melting point. This is due to the increased strength of the intermolecular forces—from London dispersion to dipole-dipole interaction because of the increased polarity. The molecules in the following show some of the example of alkyl halides:
Alkyl halide can be prepared from alcohol by reacting them with a hydrogen halide, HX (X=Cl,Br, or I). The mechanism of acid catalyzed substitution of alcohols are termed SN1 and SN2, where “S” stands for substitution while sub-“N” stands for nucleophilic, and the number “1” and “2” is described as first order and second order respectively. The “1” or “2” is also represent the reaction is unimolecular or bimolecular reaction. The secondary alcohols are more favor to react with hydrogen halides by both SN1 and SN2 mechanisms. For primary or methyl alcohol, both molecules undergo SN2 mechanism while tertiary alcohol undergoes SN1 mechanism.
R3COH > R2CHOH > RCH2OH > CH3OH
Tertiary alcohols react readily with HX alone to form alkyl halide, while secondary and primary require catalyze in the halohydrogenation reaction. Zinc chloride acts as the catalyze in the reaction. In some condition, heat supply is needed in the reaction. The mechanism for SN1 and SN2 are shown in the diagram 1.
Diagram 1
In an SN1 reaction, the protonated alcohol, or oxonium ion losses a water molecule to form a carbocation intermediate in the rate-determining step. The carbocation is then rapidly attacked by halide ion (X-) to form alkyl halide. Since tertiary alcohols form more stable carbocation intermediates than do primary and secondary alcohols, tertiary alcohols are the most likely follow the SN1 pathway.
In SN2 reaction, the nucleophile (X-) assists in the explusion of H2O from the oxonium ion via a bimolecular transition state. The SN2 process is expected to be especially slow and even is not be observed for tertiary alcohols since the transition state will be particularly crowded; as the degree of substitution decreases at the reacting center the rate of the SN2 process becomes greater and the rate of the SN1 process decreases (vide supra). Consequently, the SN2 process is the predominant one for primary alcohols.
In this experiment, t-butyl chlorride is synthesized from 2-methyl-2-propanol (t-butyl alcohol) by using HCl as the hydrogen halide. The chemical equation below show the formation of t-butyl chloride:
Diagram 2
The presence of a tertiary alkyl halide can be determined by reacting a small amount of the product with a silver nitrate (AgNO3) in ethanol. Tertiary alkyl halides will react rapidly via SN1 mechanism with the AgNO3 to form a precipitate of AgCl:
Diagram 3
To promote the above SN1 reaction, a highly polar solvent (ethanol) is used to dissolve the alkyl halide. The chloride will ionize to the alkyl cation and chloride ion. The cation will react with the alcohol solvent to form the ether and HCl. In this case both products are soluble; however, if silver ion is present in the solution, insoluble AgCl will form and a precipitate will be visible. Primary halides do not react in this test, and secondary reacts only slowly with heating.
Apparatus: separatory funnel, Erlenmeyer flask
Materials: 2-methyl-2-propanol (t-butyl alcohol), conc. HCl, saturated aqueous NaCl, saturated aqueous NaHCO, anhydrous calcium chloride, silver nitrate (AgNO3)
Procedure:
1. 5 ml of 2-methyl-2-propanol (t-butyl alcohol) is put in an Erlenmeyer flask, the flask is put over a stir motor with stir bar and commence stirring.
2. 13mL of concentrated HCl is added into the flask.
3. The mixture is stirred for 15 minutes.
4. The mixture is transferred to a separatory funnel and allowed to stand until two clear layers have separated.
5. The aqueous layer is removed and the organic layer is washed with 6mL of saturated aqueous sodium chloride solution, then with 6mL of saturated aqueous sodium bicarbonate solution and finally with another 6mL of saturated aqueous sodium chloride solution.
6. The organic layer is saved and dried with anhydrous calcium chloride.
7. The product is weighed and volume is measured to determine the yield.
Silver nitrate test: A few drops (1cm3) of your product is put into a small test tube. 2 drops of silver nitrate test solution is mixed. The appearance of a white precipitate indicates that a reaction has taken place between the alkyl halide and silver nitrate.
Result and calculation:
Observation: A white precipitate is formed after adding of five drops of silver nitrate.
Weight of conical flask = 46.6443g
Wight of conical flask + weight of t-butyl chloride = 48.7942g
Weight of t-butyl chloride = 2.1499g
Density of 2-chloro-2-mehtylpropanol = 0.7809 g/cm3
Weight = density x volume
Weight of t-butyl alcohol = 0.7809 g/cm3 x 5 cm3
= 3.9045 g
Number of mole of (CH3)3COH used = 3.9045 g / 145.072 g mol-1
= 0.02691 mole
(CH3)3COH (aq) + HCl (aq) à (CH3)3CCl (aq) + H2O (l)
1 mole of (CH3)3COH produces 1 mole of (CH3)3CCl
0.02691 moles of (CH3)3COH produces 0.02691 mole of (CH3)3CCl
Theoretical weight of (CH3)3CCl = 0.02691 mol x 163.522 g mol-1
= 4.4004g
Percentage yield = ( experimental value/ theoretical value) x 100%
= ( 2.1499g / 4.4004g ) x 100%
= 48.86%
Discussion:
In this experiment, 2-methyl-2-propnanol (t-butyl alcohol), (CH3)3COH is converted to 2-chloro-2-methylpropane (t-butyl chloride), (CH3)3CCl. In order to synthesis t-butyl chloride from t-butyl alcohol, hydrogen chloride is used to react with it. During the reaction take places, the t-butyl alcohol undergoes first order nucleophilic substitution, SN1 mechanism since t-butyl alcohol is a tertiary alkyl group. The tertiary alkyl group will not undergo second order nucleophilic substitution, SN2 mechanism. This is because the tertiary alkyl groups are successively more hindered as compared to the primary alkyl, thus this resulting in successively slower SN2 reactions.
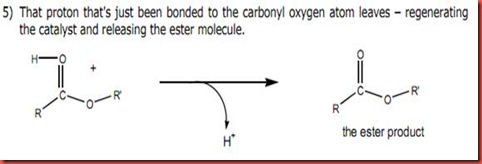
The first order rate reaction where the rate of formation of t-butyl chloride is dependent only on the concentration of the alcohol, however it is independent of the amount of acid (HCL) used. The strong concentrated hydrochloric acid (HCl) added to the t-butyl alcohol is used to provide an acidic medium and hence this protonates the electron rich hydroxyl group (nucleophile) allowing it leave as a molecule of water as shown in the diagram below:
The above diagram shows that the hydroxyl group is substituted by chloride atom when HCl is introduced.
The SN1 mechanism that t-butyl alcohol undergoes is shown in the diagram 4 below:
Diagram 4
In the diagram 4, the t-butyl alcohol acts as a nucleophile which attacks the proton from the hydronium ion in the solution. According to Bronsted-Lowry Theory, the t-butyl alcohol is considered as a base in this reaction. This is because it accepts a proton from the hydronium ion and hence t-butyloxonium ion is formed. In order to become a stable molecule, the bond between the carbon and oxygen of the t-butyloxonium ion breaks heterolytically. The breaking of bond between carbon and oxygen leads to the formation of a carbocation and a leaving group of water.
Diagram 5
As shown in the diagram 5, the carbocation is formed and it is acts as eletrophile which is the species lack of electron. Due to the lacking of electron, another nucleophile, chloride ion, Cl-, tends to attack the carbocation and hence to achieve a stable molecule. The carbocation acts as a Lewis acid which accepts electron from the chloride ion, Cl- to form t-butyl chloride. The formation of t-butyl chloride is synthesized via SN1 mechanism is shown.
The addition of concentrated hydrochloric acid into the t-butyl chloride causes the formation of cloudy solution is formed when stirring. The reaction between t-butyl alcohol and hydrogen chloride is a simple reaction which can take place in the room temperature. Two layers are formed after transferring the mixture into a separatory funnel.
The upper layer is t-butyl chloride whereas the lower layer is the aqueous layer. A 6mL of saturated sodium chloride solution is introduced into the separatory funnel after the aqueous layer is being removed. The purpose of adding concentrated sodium chloride is to pull water away from the organic layer. In another word, the saturated aqueous solution of sodium chloride is an inexpensive drying agent that will remove the bulk of water from a wet organic solution. Saturated sodium chloride can also help decrease the solubility of an organic compound in an aqueous solvent.
Aqueous sodium bicarbonate solution is added into the organic to neutralize the acidic medium that caused by concentrated hydrochloric acid added. Since sodium bicarbonate is an alkaline solution. The neutralization process between sodium carbonate and hydrochloric acid could be shown in the following chemical equation.
NaHCO3 (aq) + HCl (aq) --> NaCl (aq) + H2O (l) + CO2 (g)
The sodium chloride salt, water, and gaseous carbon dioxide are formed in the neutralization process. The two layers are formed second time due to the formation of water in the neutralization. The sodium chloride is highly soluble in aqueous layer which is being discarded together with aqueous layer. Another portion of 6mL of saturated sodium chloride solution is introduced into the separatory funnel, to isolate the organic layer from aqueous layer left and reduce the solubility of the organic layer in water. Finally, the drying agent, anhydrous calcium chloride is added to remove all the water droplets in order to obtain a dried organic layer. Excess anhydrous calcium chloride is highly recommended to be used to make sure that there is no water droplet inside. The chemical reaction of anhydrous calcium chloride with water is
CaCl (s) + H2O (l) --> CaCl.nH2O (s)
Anhydrous Hydrated
calcium chloride drying agent
The presence of tertiary alkyl halides can be tested by using silver nitrate test. Some of the product formed in the experiment is added with silver nitrate solution. The observation we obtained is a white precipitate is formed after addition of silver nitrate solution. This is because the t-butyl chloride containing tertiary alkyl group which reacts rapidly via SN1 mechanism with the silver nitrate to form a precipitate of silver chloride.
As shown in the diagram above, a highly polar solvent (ethanol) is used to dissolve the butyl chloride. The chloride will ionize to the butyl cation and chloride ion. The butyl cation will react with the alcohol solvent to form the butyl ethyl ether via formation of C-O bond. The HCl is formed in this reaction too. In this case both products are soluble; however, if silver ion is present in the solution, insoluble AgCl will form and a precipitate will be visible. Primary halides do not react in this test, and secondary reacts only slowly with heating.
![clip_image002[4] clip_image002[4]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEhEPVYqocE3sLWXqUBT185bvpygUyEDWVFYzZnqUHlA_5Wet5bFJhXyCRXG1VxKTgjiklgMdQePrIxbVeNDuw1_ipysyDeP7mkGXnohJpMzFxp70TxOfZn85QKsFs7DFAO5EwTCSpe75yGU/?imgmax=800)
![clip_image006[4] clip_image006[4]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEioz40kWMelFtSipKc4iEl8K8mA5-o8lYziZbUiA1NyZU6paVkk8WL6-eL5SUWrVxgCmEqPc5kQvbcDSSvXcx5WchmFnPi8m8kGujoDnwAsL7WXCUfHMiXtFqLZwC9VP185ByhJxyTkwi_b/?imgmax=800)
![clip_image008[4] clip_image008[4]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEjx4fX88ty7_IUTGO00srGWgjI3_uVAkotrqfbUfJLuu44jvaSNBjS_GuH4dF9Wm5yFLbtOr1Y4jIefCtLz8-NOcjsIF6-uDZGoma2TWdDYhRZrcewguk-m1VrxxK2EHJ0q8k9oq2s0NO3P/?imgmax=800)
![clip_image010[4] clip_image010[4]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEhEXJZVy9oKJnu1L4kKRSrvNz6jmdJCnrOgB2cxJqwzOiqiYyreHhnpI1UpWsEioxlsN2_DqS3xdG4RzZsGLMOsLANO39yWh-XZIJ3s7fwhK1inN8832FGPmTZb30Dk7oyGrCPqG2DQpWqW/?imgmax=800)
![clip_image012[8] clip_image012[8]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEg3wFq3fkFBsB_Px010MXFH1ZCQVIyOkw21Q1kysNmwpBjm0AfhpxKYLNoCxwhglJT7xKoSfFAdjhVG8tIRWrVkBcmcGYXh0okIwIr9qAovxNwj3b1_kXCmSBvY-sphahR_9on8Bxu3C-NY/?imgmax=800)

![clip_image016[4] clip_image016[4]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEhvh0StW-osjF5fhz3G-lSpsxqaMRsktLPkd0q2wy4NQ6w_5qBc55etfpsnUgNy0OwoaljM5cwDIyU67aXIqlMGqaXiXX8N_qE-i5WiDCP4bs8RSqFmkiAGss7fcSOnburneb1W9qvwSCJG/?imgmax=800)















![clip_image002[5] clip_image002[5]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEi0NHLWEhjUL-s8OW9lfZyo1yr0OcctiIl-kwTSzhCLDPRdEPs9qc0ZA8sUNIqoMwi8C7IVrowpardL7dMXAHs1pMajJbl8jEHa1G1XjFu8ts3UU5eAL7tpOHrxWrrG97TQmOyMQwbox0JM/?imgmax=800)




![clip_image031[5] clip_image031[5]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEhpeDmiIjpCA5PDKuGO7HK7ZGaeAoFLkHgTBSiGNftH0Xc177w08EIqqgsKQPND3r5RzA0Z1ziagQMwpTKp3Za5mSZJbDT8HKPfpHxfcELns3GQEYviK_DeXaTer9u3NgBTpY1tFPWGx026/?imgmax=800)
![clip_image033[5] clip_image033[5]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEjWkbZwuEgozcVT4jWfQEKgbgq34a3bFRKL1NfgjMwvUC2TVsyhwPN4bOb80Iz7FkPKREQnQR3Sa5gcx1s80JaAr0X80hHlT6uqkrt11MXxQn56hnqzmjDg_1YOfa-dw5VzpW576uPzv8wN/?imgmax=800)
![clip_image035[5] clip_image035[5]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEiPZfpRWpoIRCekwGOI23qPfS-HiN3-5z-guY3s2kvtH1Ws1ygfQS8GH0L2yjLSQbRwZ_-YHgYm8ZjekC1TFVQ55kePQYS94USj_uvfAow9julHvtplDGXci0JO80WyHp8FzHN-LRktRW_F/?imgmax=800)

