1. To prepare tris(acetylacetonato)manganese(III), Mn(acac)3 {also called tris(2,4-pentanedionato)manganese(III)}
2. To identify and draw the structure of Mn(acac)3
3. To find out the magnetic moment of Mn(acac)3
Intorduction:
A coordination complex or metal complex is a structure which consisting of a central transition metal is bonded to one or more as ligands (neutral or ionic). Ligand is defined as the molecule or ion that is attached directly to the central metal atom or ion through dative covalent bond which both bonding electrons are contributed by ligand. The metal is Lewis acid which is being an electron pair acceptor while the same or different types of ligands are being the Lewis bases which donate their electron pair to central metal. The bond between metal and a ligand is a Lewis acid-base interaction.
When a ligand is bounded to a central metal through a single donor atom, then the ligand is known as unidentate (the ligand binds to the metal through a single point of attachment as it has one tooth). For a bidentate ligand, it can use two atoms donor to bind to a metal. Larger ligands may contain more than one capable of coordinating bonds which bonded to a single central metal. These kind of ligands are known as polydentate. When a bi- or polydentate ligand uses two or more donor atoms to bind to a single metal ion, it is said to form a chelate complex. Such complexes tend to be more stable than similar complexes containing unidentate ligands due to chelating effect.
The coordination number of a metal complex is determined directly from the number of ligand that can be accepted by a transition metal. In order to obtain the oxidation state of the metal in the complex to be synthesized, this may need prior oxidation or reduction of the starting material. The metal salt, manganese(II) chloride tetrahydrate is the starting material in this experiment. In the presence of a base, the acetylacetone is readily to lose a proton to form the acetylacetone anion, acac- through monodeprotonation of acetylacetone. Hydrogen atoms on the carbon atoms that are adjacent to carbonyl (C=O) groups are relatively acidic. In the isomerism, the acetylacetonate ion has three resonance forms. This ligand is a bidentate which used its two oxygen atoms to coordinate by donating lone pair electrons towards central metal. The diagram 1 below shows the structure of acetylacetonate ion.
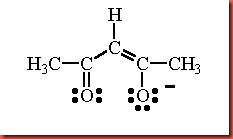
Diagram 1 Structure of acetylacetonate ion
In this experiment, the manganese complex is a good example of octahedral complex which containing three bidentate ligands. The metal ion is electrically neutral because it is carrying a 3+ charge and each ligand carried -1 charge. The complex is more soluble in organic solvent than water due to its neutral properties. Manganese exhibits an unusually wide range of oxidation state which includes +7 to -1, but in aqueous solution the +2 oxidation state is the most common. The three bidentate ligands are pack very efficiently around the trivalent ions of transition metal. As a result, the Mn2+ can be easily to be oxidized to form Mn(acac)3 in the presence of acetylacetonate ions. Oxidizing agent (potassium permanganate solution) is used to oxidize the four equivalent of Mn2+ to become Mn3+ as shown in the equation below:
MnO4- + 4Mn2+ + 8H+ à 5Mn3+ +4H2O
The formation of Mn3+ ions are used to react with acetylacetonate ions to produce Mn(acac)3. At the same time, the deprotonation of acetylacetone will produce a large amount of H+. In order to maintain the acidity of the solution, the sodium acetate is added to neutralize the acid released which proton reacts with acetate ion to form weak acetic acid.
7H+ + 7CH3COO- à7CH3COOH
Transition metals have variable oxidation state. In their complexes, the number of outer valence electrons varies with the oxidation state. Since the electrons spin and generate a magnetic field, the magnetic properties of transition metals in their complexes are of great interest in determining the oxidation state, electron configuration and so on. The magnetic moment of the metal is determined indirectly from the magnetic susceptibility. The unit measurement for magnetic moment is Bohr magneton, μB. Apparatus:
Conical flask
Measuring cylinder
Glass rod
Hot-plate stirrer and magnetic bar
Thermometer
Beaker
Filter funnel
Suction filter
Materials:
Manganese(II)chloride tetrahydrate
Sodium acetate trihydrate
Acetylacetone
Potassium permanganate solution
Acetone
Procedure:
1) 5g (0.025 mol) of manganese(II) chloride and 1.3g (0.0095 mol) sodium acetate trihydrate were dissolved in 200ml of distilled water. The stirred solution was added slowly with 21cm3 (~0.20mol) of acetylacetone(check whether its purity is 99%, density 0.975g cm-1; record the actual purity and density of the acetylacetone).
2) The resultant two phase system was treated with potassium permanganate solution (1g in 50cm3).
3) After a few minutes, small amounts of sodium acetate solution were added with stirring (13g or 0.095 mol of sodium acetate trihydrate).
4) The solution is heated to about 60°C with continuous stirring for 30 minutes.
5) The resultant solution was cooled in the ice-cold water and then the solid complex formed was filter by suction filtration. The complex was washed with acetone and was sucked until dry.
Result and calculation:
Weight of manganese(II) chloride tetrahydrate, MnCl2.4H2O = 5.0024g
Weight of first protion of sodium acetate trihydrate, Na(acac)3 = 1.3038g
Weight of potassium permanganate, KMnO4 = 1.0091g
Weight of second portion of sodium acetate tirhydrate, Na(acac)3 in 50cm3 distilled water = 13.0120g
Weight of filter paper = 0.7962g
Weight of filter paper + weight of complex = 4.9926g
Weight of complex = 4.1964g
Purity of acetylacetone = 100%
Density of acetylacetone = 0.975g/cm3
Mass of acetylacetone = density x volume used
= 0.975g/cm3 x 21cm3
= 20.475g
Molecular mass of acetylacetone = [(12.01x5) + (16x2) + (1.008x8)] g/mol
= 100.11 g/mol
Number of mole of acetylacetone used = Mass/ Molecular mass
= 20.475g / 100.11g mol-1
= 0.2045 mol
Molecular mass of manganese(II) chloride tetrahydrate
= [54.94 + 35.45(2) + 4 (18.016)] g/mol = 197.90 g/mol
Number of mole of manganese(II)chloride tetrahydrate = 5.0024g/ 197.90 g mol-1
= 0.0253 mol
Mass of Mn(acac)3 = 4.1964g
Molecular mass of Mn(acac)3
= [54.94 + 3(12.01x5 + 16x2 + 1.008x7)] g/mol= 352.258 g/mol
Number of mole of Mn(acac)3 = 4.1964g/ 352.258g mol-1
= 0.0119 mol
No of mole of KMnO4 = 1.0091g/ 158.0339 g mol-1
= 0.0064 mol
4Mn2+ + MnO4- + 15Hacac à 4H2O + 7H+ + 5Mn(acac)3
Theoretical mass of Mn(acac)3 = 5 x 0.0064 mol x 352.2617g/mol
= 11.2724g
Percentage yield of Mn(acac)3 = 4.1964g/11.2724g x 100%
= 37.23%