Objectives:
1. To prepare both cis- and trans copper glycine complexes
2. To verify the complex is kinetic product or thermodynamic product
3. To characterize both cis- and trans copper glycine complexes
Introduction:
Glycine is one of the biologically important compounds in the group of amino acids. Among the twenty one natural amino acids, glycine is the simplest amino aicd. Amino acid has both the functional group of amine (-NH2) and carboxylic acid (-COOH). They are the basic units of the proteins in which the building block of every single living cell. Proteins are polypeptides or polyamide that formed by joining the –NH2 group of one amino acid to the –COOH group of another one and therefore a long and complicated peptide chain is formed.
Glycine is the simplest model for peptide coordination and its complexes with various metal ions have been thoroughly studied. Elzbieta (2008) claimed that glycine can even forms more essentially stable complex with copper (II) compared to other amino acids. The structure and stability of the complexes are determined by nature of metal, the nature of the ligands and environment. The environment is controlled by the factors such as temperature, the type of solvent, the interaction enthalpies, entropies, and Gibbs energies.
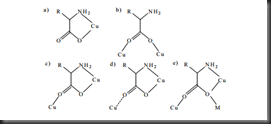
The binding modes of glycine ligand can be varied since it has at least two donor atoms. The glycinate ion is able to adopt a η3-coordinated mode via its amino, -NH2 and carboxylate, -COOH groups to chelate one copper ion and bridge to another copper ion. The variation of glycinate binding modes is shown in the picture below:
Picture 2. Five coordination modes of amino acid. a) η1-coordination mode; b)η2-coordination mode; c) η3-coordination mode with strong Cu-O bond; d)η3-coordination mode with weak Cu-O bond beyond 2.5 Å; e)η4-coordination mode (M is Na+ ion or lanthanide ion)
In this experiment, a pair of geometric isomers of copper(II) glycine complex are prepared. Deprotonated glycine, or known as glycinate ion, NH2CH2COO- is capable to form two coordination bonds to copper metal through the lone pair electrons of nitrogen and oxygen atoms. Hence, it functions as a chelating ligand or more specifically it is known as bidentate ligand and favors the formation of bis(glycinato)copper(II) complex. The reaction between copper(II) acetate monohydrate and glycine can produces mixture of both isomers in an equilibrium mixture. However, the cis isomer precipitates much more quickly then trans isomer and hence leading to a shift in equilibrium away from trans with producing only cis isomer. Cis isomer is the kinectically favoured product whereas trans isomer is thermodynamically favoured. In order to produce trans isomer, the cis isomer can be converted into another isomer by supplying heat energy at 180 °C for time of 15 minutes.
| Picture 3. a) cis-Cu(gly)2 b) trans-Cu(gly)2 |
Materials:
Copper(II) acetate monohydrate, glycine, ethanol 95%
Apparatus:
Magnetic stirring hot plate, magnetic stirring bar, Erlenmeyer flask, Hirsch funnel, test tube
Procedure:
Part A: Preparation of cis-bis(glycinato)copper(II) monohydrate
Part B: Preparation of tran-bis(glycinato)copper(II)
Results and calculations:
Table 1 Amount of reactants used and amount of products obtained
| Reactants | |
| Weight of copper(II) acetate monohydrate | 0.3007g |
| Weight of glycine | 0.2328g |
| Products | |
| Weight of filter paper I | 0.3233g |
| Weight of filter paper I + cis-product | 0.6061g |
| Weight of cis-product | 0.2828g |
| Weight of filter paper II | 0.3001g |
| Weight of filter paper II + trans-product | 0.3684g |
| Weight of trans-product | 0.0683g |
Chemical reaction:
(CH3COO)2Cu.H2O + 2 H2NCH2COOH àcis-(H2NCH2COO)2Cu.H2O + 2 CH3COOH
Determination of limiting agent
Molecular weight of (CH3COO)2Cu.H2O = 199.55 g mol-1
Number of mole of (CH3COO)2Cu.H2O = 0.3007g / 199.55 g mol-1
= 0.0015 mol
Molecular weight of H2NCH2COOH = 75.00 g mol-1
Number of mole of H2NCH2COOH = 0.2328g / 75.00 g mol-1
= 0.0031 mol
Thus, copper(II) acetate monohydrate is the limiting agent in this reaction.
Percentage yield calculation
Molecular weight of (H2NCH2COO)2Cu.H2O = 229.55 g mol-1
Theoretical weight of cis-(H2NCH2COO)2Cu.H2O = 0.0015 mol x 229.55 g mol-1
= 0.3443 g
Percentage yield of cis-(H2NCH2COO)2Cu.H2O = 0.2828g / 0.3443g x 100%
= 82.14%
Theoretical weight of trans-(H2NCH2COO)2Cu. = 70 mg
Percentage yield of tran-(H2NCH2COO)2Cu = 0.0683g / 0.070 g x 100%
= 97.57%
Discussion:
From this experiment, the weights of both isomer cis-Cu(gly)2.H2O and trans-Cu(gly)2 obtained are 0.3443g and 0.0683g respectively. Each isomer contributed the percentage yield of 82.14% for cis-Cu(gly)2.H2O and 97.57% for trans-Cu(gly)2 respectively.
The dissociation of glycine molecule produces a glycinate anion, NH2CH2COO- in which it replaces the position of acetate ion, CH3COO- in the copper complex. The dissociated proton from glycintate ion is accepted by acetate ion and hence acetic acid is produced in the reaction between copper(II) acetate monohydrate and glycine.
(CH3COO)2Cu.H2O + 2 H2NCH2COOH à(H2NCH2COO)2Cu.H2O + 2 CH3COOH
The reaction was takes place in a hot 95% ethanol. Ethanol did not participate in the reaction but it acts as a medium to allow the copper(II) complex to form at 70°C. When cooled down, the copper(II) complex crystallize out from the ethanol.
The solid cis-monohydrate was heated at 200 °C by using an aluminium block in order to convert cis-monohydrate to trans-complex. The temperature of aluminium block is approximately measured by using hexane with a thermometer. The dehydration of cis-bis(glycinato) copper(II) monohydrate, cis-Cu(gly)2.H2O at sufficiently high temperature (approximately at 200 °C)leads to the formation of mainly anhydrous trans-complex, which is readily to be re-hydrated to give trans-bis(glycinato) copper(II), trans-Cu(gly)2.H2O if present in solution.
Precaution steps:
1. Handle copper(II) acetate monohydrate carefully. It is harmful if swallowed, inhaled or absorbed through the skin.
2. Keep a distance from the hot plate when heating aluminium block.