Questions:
1. Calculate the polymer solubility parameter, δ2 for PVC, LDPE, PS and PMMA by using the equation below, where the molar attraction constant per unit for the functional groups are referring to the appendix 1.
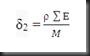
| Type of polymers and their repeating unit | Molecular weight, M (g mol-1) | Sum of molar attraction constant, ∑E (J1/2 cm3/2 mol-1) | Density, ρ (g cm-3) | Solubility parameters, δ2 (J1/2 cm-3/2) |
| -(CH2CHCl)- Poly(vinyl chloride) | 62.50 | 891.00 | 1.41 | 20.10 |
| -(CH2CH2)- Low Density Polyethylene | 28.00 | 560.00 | 0.85 | 17.00 |
| -CH2CHΦ)- Polystyrene | 104.00 | 1937.00 | 1.05 | 19.56 |
| -[CH2CH(COOCH3)]- Poly(methyl methacrylate) | 86.00 | 1352.00 | 1.17 | 18.39 |
2. Explain how solubility is affected by polymer chain structure.
When molecular weight of polymer increases, its solubility will decrease. Crosslinked polymers do not dissolve but they only swell when the solvent diffuse into the polymers. Lightly crosslinked polymers swell extensively in solvent in which unvulcanized material would dissolve. When crosslinking degree increases, the solubility decreases. This is because the strong cross-linked polymers will inhibit interaction polymer chains and solvent molecules.
No comments:
Post a Comment