Asymmetric Synthesis with Baker’s Yeast:
Objectives:
1. To reduce 1-phenyl-1,2-propanedione to 1-phenyl-1,2-propanediol
2. To monitor the course of reaction by using TLC
3. To characterize the produce by FT-IR spectroscopy and gas chromatography-mass spectroscopy (GC-MS)
Introduction:
Lithium aluminium hydride and sodium borohydride are good reducing agent, in which it can reduce a ketone to an alcohol. However, they are not able to generate a chiral alcohol because they can nucleophilic attack both sides of carbonyl group, hence producing racemic mixture. In order to obtain a chiral alcohol, baker’s yeast is used.
Asymmetric reduction of 1-phenyl-1,2-propanedione by using baker’s yeast in this experiment in order to produce (-)-(1R,2S)-1-phenyl-1,2-propanediol with the enantiomeric excess of 98% or more. The completeness of reaction is determined by using thin layer chromatography (TLC). Pure 1-phenyl-1,2-propanediol is characterized by using infrared (IR) spectroscopy and mass gas chromatography-mass spectroscopy (GC-MS).
Apparatus:
Erlenmeyer flask, hotplate-stirrer, magnetic stirring bar, TLC tank, micropipette, separatory funnel, UV lamp, rotary evaporator
Materials:
1-phenyl-1,2-propanedione, freeze-dried Baker’s yeast, TLC plate, tert-butyl methyl ether (BME), magnesium sulphate anhydrous, cylohexane
Instruments:
IR spectroscopy, gas chromatography-mass spectrometer
Procedures:
Result and calculations:
Observations on TLC plate:
|
Table 1: Rf value of each spots on TLC plate
| Time (minutes) | 0 | 20 | 40 | 60 | |
| Distance travelled by each aliquots (cm) | 1st spot | 2.90 | 2.90 | 2.90 | 2.90 |
| 2nd spot | 5.20 | 5.20 | - | - | |
| Retardation factor, Rf | 1st spot | 0.41 | 0.41 | 0.41 | 0.41 |
| 2nd spot | 0.74 | 0.74 | - | - |
*TLC is performed by using a mixture of cyclohexane:BME (3:2)
*solvent front is 7cm.
Table 2: Weight of 1-phenyl-1,2-propanediol
| Weight of round bottom flask | 97.6703g |
| Weight of (round bottom flask + 1-phenyl-1,2-propanediol) | 97.9018g |
| Weight of 1-phenyl-1,2-propanediol | 0.2315g |
Table 3: Significant peaks of starting material and product in IR spectrum
| Functional group | Wavenumber of Compound, v (cm-1) | |
| 1-phenyl-1,2-propanedione | 1-phenyl-1,2-propanediol | |
| C=O stretch | 1715, 1676 | absent |
| O-H stretch | absent | 3369 |
Molecular weight of 1-phenyl-1,2,-propanedione = 148 g /mol
Molecular weight of 1-phenyl-1,2-propanediol = 152 g / mol
Density of 1-phenyl-1,2,-propanedione = 1.101 g ml-1
Mass of 1-phenyl-1,2,-propanedione = density x volume
= 1.101 g ml-1 x 0.23 ml
= 0.2532g
1 mole of 1-phenyl-1,2,-propanedione produces 1 mole of 1-phenyl-1,2-propanediol
Mole number of 1-phenyl-1,2,-propanedione = 0.2532g / 148 g mol-1
= 0.0017 mol
Theoretical mass of 1-phenyl-1,2-propanediol = 0.0017 mol x 152 g mol-1
= 0.2584g
Percentage yield = 0.2315g / 0.2584g x 100%
= 89.59%
Discussion:
In this experiment, 1-phenyl-1,2-propanedione was reduced to 1-phenyl-1,2-propanediol by using baker’s yeast. The mass of 1-phenyl-1,2-propanediol obtained experimentally is 0.2315g and its percentage yield is 89.59%.
Thin layer chromatography was used to monitor the reduction of 1-phenyl-1,2-propanedione in this experiment. Based on the observation of TLC plate, the reaction was completed at 40th minutes since the TLC plate only shows one spot is present. After the reaction completed, the product was extracted with BME and was dried with drying agent to remove water in the organic layer.
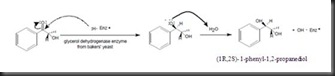
The following mechanism shows that how ketone was reduced into alcohol by yeast.
Firstly, the hydride from baker’s yeast nucleophilic attack to carbonyl carbon (labeled as carbon 2) and caused the reduction of the carbonyl group. Oxygen of the particular carbonyl group was bearing with partial negative charge in which it can abstract a proton from water molecules to form hydroxyl group.
From the IR spectrum, 1-phenyl-1,2-propanedione shows the presence of C=O stretch at 1715cm-1 and 1676cm-1. Besides, this ketone compound did not show any O-H stretch signal in the IR spectrum. From the alcohol spectrum, there shows a broad O-H stretch signal at 3369cm-1 and it did not have C=O stretch signal. According to IR spectrum, the ketone compound has been successfully reduced to form an alcohol compound as obtained in the experiment because IR spectrum indicated the presence of O-H group.
Precaution steps:
1. Do not use separatory funnel point to anybody when releasing the vapour.
2. Do not shake the separatory funnel vigorously to avoid emulsion formation.



No comments:
Post a Comment