Objectives:
1. To identity the types of polymer by using water
2. To identity the types of polymer by using copper wire
3. To identity the types of polymer by using alcohol
4. To identity the types of polymer by using acetone
5. To identity the types of polymer by using oil
6. To identity the types of polymer by using heat
Introduction:
Polymers are long chain organic molecules that are assembled from many identical and smaller molecules called monomers. Polymerization of is the process to assemble all the monomers together in order to form a huge and complicated molecule.

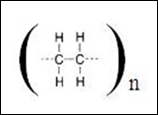
Polymer is containing many units of monomer in its long chain. When there is a lot of monomer joining together chemically, polymer is formed, which shown in the picture below:

Generally, we can use the symbol below to show the structure of polymer instead of drawing the whole structure of polymer. Besides, we are not able to draw the whole structure because the number of monomer is usually over hundreds or even thousands of units.
The above picture refers to the simple structure in which it shows the whole structure of polymer or we named it as molecular formula as well.
The polymers shown in the picture above are the most common polymer that we always see and use in daily life. These includes polyethylene terephthalate (PETE), high density polyethylene (HDPE), polyvinyl chloride (PVC), low density polyethylene (LDPE), polypropylene (PP) and polystyrene (PS). These polymers are classified as class 1, followed by class 2, class 3 and so on. Identification of polymer by simply observing its appearance is difficult to differentiate them. However, we can identify them by using water, copper wire, alcohol, acetone, oil and heat.
Lassaigne's Test or Sodium Fusion test is also can be used to identify some of the polymers. For example, the presence of nitrogen, N in polyamides, and the presence of chlorine, Cl in PVC can be identified by using sodium fusion test. Besides, IR also can be used to identify the type of polymer via their major functional groups. The carbonyl stretches, O-H stretches, aromatic bends, etc could clearly show the identity of a polymer. Despite these test can be applied in identifying polymers, but today we only focus on the simplest test.
In this experiment, we will use some known plastic material and carry out some tests to identify the types of polymer. The flow chart below shows the overall tests on polymer identification.
We will use another three unknown sample of plastic to perform the test on them in order to identify the unknown samples.
Material:
Samples of polyethylene terephthalate (PETE), high density polyethylene (HDPE), polyvinyl chloride (PVC), low density polyethylene (LDPE), polypropylene (PP) and polystyrene (PS), three unknown samples of plastic, isopropyl alcohol, corn oil, copper wire, acetone
Apparatus:
Test tube, test tube rack, stopper, Bunsen burner, beaker, forceps, gauze wire
Procedure:
A) Water test
1. Pour 10ml of water into a test tube.
2. Place one of the samples into the water and stir the water by using a glass rod.
3. Observe whether the sample sink or float on the water surface.
4. Remove the sample from water and dry it for later use.
5. Repeat steps 1 to 4 by using each of the samples.
6. Take the samples that sank in the water for copper wire test while save the floated sample for alcohol test.
B) Copper wire test
1. Obtain a piece of copper wire about 6cm long and insert it into a small cork.
2. Burn the copper wire by using a Bunsen burner and heat until it is red hot.
3. Remove the copper wire from flame and touch on the sample of plastic. A small amount of sample should melt.
4. Place the wire copper with sample into the flame. A luminous flame is observed.
5. Repeat steps 1 to 4 by using the samples which sank in the water.
C) Acetone test
1. Prepare 20ml of acetone into a beaker under the fume hood.
2. By using a forceps, place the sample of plastic into the acetone for 15 seconds.
3. Remove the sample and press it firmly.
4. Scrape the sample to observe if the outer layer has softened.
5. Repeat steps 1 to 4 on the sample that gave yellow-orange flame.
D) Heat test
1. Prepare 100ml of water in beaker and heat it until boil.
2. By using a forceps, place the sample of plastic into the acetone for 15 seconds.
3. Remove the sample and press it firmly.
4. Scrape the sample to observe if the outer layer has softened.
5. Repeat steps 1 to 4 on the sample that shown negative result in acetone test.
E) Isopropyl alcohol test
1. Pour 10ml of isopropyl alcohol into a test tube.
2. Place one of the samples samples that floated on the water surface into the test tube and stir the water.
3. Observe whether the sample sink or float on the surface.
4. Remove the sample from water and dry it for later use.
5. Repeat steps 1 to 4 by using the samples that floated on the alcohol surface.
F) Oil test
1. Pour 10ml of oil into a test tube.
2. Place samples that floated on the alcohol surface into the test tube and stir the sample.
3. Observe whether the sample sink or float on the surface.
4. Repeat steps 1 to 4 on the unknown samples
Precaution steps:
1. Carry out the test under a fume hood.
2. Avoid the isopropyl alcohol and acetone from any sources of flame.
3. Hold the wire copper by using a forceps after heated.
4. Equip with personal protective equipment such as glove and mask.


Hi there, I found your web site via Google while looking for a related topic, your website came up, it looks good. I have bookmarked it in my google bookmarks handbook of clinical sexuality for mental health professionals
ReplyDeletelinkedin
ReplyDeletelinkedin
linkedin
tlinkedin
linkedin
linkedin