Objectives:
1. To synthesize a cobalt hydride complex
2. To deduce its chemical structure based on the spectral data
Introduction:
Metal hydride complexes are very crucial as the intermediates in many catalytic processes such as alkene oligomerization and hydrogenation. Covalently bonded metal hydride complexes are known for all the transition metals. The complexes often contain the metal in a low oxidation state with the ligands of phosphines, carbon monoxide or cyclopentadiene.
Isomerization of alkene is always a possibility in any homogenous catalytic reaction that involved alkene. Migratory insertion of alkene into the metal hydrogen (M-H) bond can occur in a Makovnikov addition or anti-Makovnikov manner. Alkene isomerization is a process that involves Makovnikov addition followed by a β-elimination which is shown in the diagram 1 below:
| Figure 1: Isomerization of butene by hydride mechanism |
In the Figure 1, it shows that the but-2-ene is synthesized from but-1-ene through the hydride mechanism in which metal hydride acts as the intermediate.
One of the well known processes of homogeneous catalytic reaction is hydrogenation of alkene. The metal hydride complex plays a very important intermediate in the hydrogenation of alkene, for example, Wilkinson’s catalyst in the hydrogenation of alkene. The following figure 2 shows how the metal hydride acts as an intermediate in the particular process.
Firstly, Wilkinson’s complex will dissociates one phosohine ligand to form a 14 electron complex. This followed by the oxidative addition of hydrogen to form a metal hydride intermediate. In the following step, alkene is added into the metal complex via ligand addition. Migratory insertion of alkene into the M-H bond leads to the formation of alkyl ligand that bonded to rhodium metal. Alkane is formed at the end of the process via reductive elimination between a hydride and an alkyl group. The catalyst is being reused in the hydrogenation process and the process is repeating again.
In this experiment, metal hydride complex is being synthesized and characterized by using proton nuclear magnetic resonance (1H NMR) spectrophotometer in order to determine the number of proton present. IR spectrophotometer is also used in characterizing the metal complex.
Materials:
Sodium borohydride, ethanol, cobalt(II) nitrate hydrate, triphenylphoshate, methanol, dichloromethane
Instruments:
FT-IR spectrophotometer, NMR spectrophotometer
Apparatus:
Melting point apparatus, hotplate stirrer, magnetic stirrer bar, Buchner funnel, beaker, Erlenmeyer flask, Hirsch funnel
Procedure:
Part A: Preparation of Metal hydride
Part B: Characterization of Metal hydride
Results and calculation:
Table 1: Weight of hydridotetrakis(triphenylphosphito)cobalt(I)
| Weight of empty sample vial | 12.5925g |
| Weight of ( sample vial + metal hydride) | 19.3553 g |
| Weight of metal hydride | 6.7628 g |
Calculating percentage yield of metal complex
One mole of Co2+ reacts with one mole of P(C5H5)3.
Mole number of Co2+ = 1.5240g / 200.93g mol-1
= 0.0076 mol
Mole number of P(C6H5)3 = 6.8037g/ 261.97g mol-1
= 0.026mol
Thus, Co2+ is the limiting reagent since P(C6H5)3 is in excess.
Theoretical mass of hydridotetrakis(triphenylphosphito)cobalt(I)
= 0.0076mol x 1107.81g mol-1
= 8.4194g
Percentage yield = 6.7628 g / 8.4194g x 100%
= 80.32%
| Value of integration | = 60 | = 1 |
| Ratio | 60 | 1 |
| Number of protons present | 60 | 1 |
Note: One triphenylphosphate contains 15 H
Since one triphenylphosphate contains 15H, so the presence of 60H indicates that there is four triphenylphosphate ligands binded to the metal hydride complex.
Table 2: Significant peaks of cobalt(II) nitrate hydrate in IR spectrum (Appendix I)
| Significant signals | Wavenumber (cm-1) | |
| Expected (from table) | Observed (from spectrum) | |
| O-H stretch | 3200-3550 | 3403 |
| Asymmetric NO2 stretch | 1450-1600 | 1629 |
| Symmetric NO2 stretch | 1260-1375 | 1384 |
Table 3: Significant peaks of triphenylphosphite in IR spectrum (Appendix II)
| Significant signals | Wavenumber (cm-1) | |
| Expected (from table) | Observed (from spectrum) | |
| Aromatic C=C stretch | 1400-1600 | 1481, 1590 |
| =C-H stretch | 3010-3100 | 3062, 3038 |
| C-P stretch | 700 | absent |
Table 4: Significant signals of hydridotetrakis(triphenylphosphito)cobalt(I) in IR spectrum (Appendix III)
| Significant signals | Wavenumber (cm-1) | |
| Expected (from table) | Observed (from table) | |
| =C-H stretch | 3010-3100 | 3067 |
| Aromatic C=C stretch | 1400-1600 | 1490, 1591 |
| Co-H stretch | 1745-1933 | absent |
| C-P stretch | 700 | 691 |
Note: The C-P stretch value is obtained from journal. (Kurita et al, 2003)
Electron counting of hydridotetrakis(triphenylphosphito)cobalt(I)
Hydride: donates 2 electrons, each triphenylphosphito donates 2 electrons
Hydride: -1, triphenylphosphosphito: neutral
Oxidation state of cobalt = Co(I), with d6
Total electron count = 2 + 4(2) + 6
= 16 electrons
Discussion:
The percentage yield of hydridotetrakis(triphenylphosphito)cobalt(I) is 80.32% in which the mass of product obtained experimentally is 6.7628 g.
In this experiment, sodium borohydride (white) was used to provide hydride to metal complex to form a cobalt hydride complex. Ethanol acts as a medium to allow the cobalt complex (greenish brown) can form in the solid state since the cobalt hydride complex is not soluble in ethanol. The product was washed with ethanol, water and methanol is to remove any other unreacted starting materials. This method can reduce the presence of impurities.
From the IR spectrum of triphenylphophate, the significant signals include aromatic C=C (1481cm-1 and 1590cm-1) and =C-H (3062cm-1 and 3038cm-1). However, the expected C-P stretch at 700cm-1 did not present. According to the IR spectrum of product, the significant signals that present are =C-H- stretch (3067cm-1), aromatic C=C (1490cm-1 and 1591cm-1) and C-P stretch (691cm-1). However, there is another expected significant signal did not present in the IR spectrum which is the Co-H stretch (1745-1933cm-1).
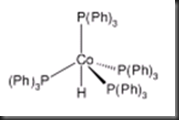
Based on the 1H NMR spectrum of product, the integration of the first signal (at positive ppm value) shows 66mm while the second signal (at negative ppm value) shows 1.1mm. From the spectrum, number of protons present in the first and second signals is 60 H and 1 H respectively. Hence, the molecular structure of hydridotetrakis(triphenylphosphito)cobalt(I) is deduced as monocapped tetrahedral with the hydride as the face-capping ligand. The figure 3 below shows the structure of hydridotetrakis(triphenylphosphito)cobalt(I).
Figure 3: Molecular structure of hydridotetrakis(triphenylphosphito)cobalt(I)
The fomal oxidation state of cobalt in the metal complex is Co(I). This is because there is only one hydride carrying one negative charge bonded to cobalt while the other four triphenylphosphine ligands are neutral. Based on the electron counting in the calculation and result part, the total electron is 16. The metal complex is stable in 16 electrons because the four sterically bulky triphenylphosphine ligands blocked the vacant site and hence other ligand is not allowed to bind to cobalt. As a result, hydridotetrakis(triphenylphosphito)cobalt(I) has a total number of 16 electrons.
Precaution steps:
1. Make sure the sodium borohydride will never contact with acid or water since it will liberates hydrogen.
2. Dichloromethane is highly volatile and must be used in the fume hood only.