Objectives:
1. To synthesize poly(methyl methacrylate) and poly(methyl methacrylate)-co-poly(butyl acrylate)
2. To characterize poly(methyl methacrylate) and poly(methyl methacrylate)-co-poly(butyl acrylate) by using infrared spectroscopy (IR) and differential scanning calorimetry (DSC)
Introduction:
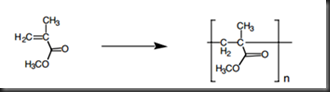
Poly(methyl methacrylate), PMMA, is known as Plexiglas in 20th century, is an amorphous, transparent and colourless thermoplastic. PMMA is hard and stiff but brittle and notch-sensitive, with a glass transition temperature of 105°C. PMMA is a polymer that made up from its basic unit of methyl methacrylate, MMA. Figure 1 show the monomer MMA and its polymer.
Figure 1 PMMA and its monomer unit
PMMA is a polymer that has characteristics of good abrasion and UV resistance and excellent optical clarity but poor low temperature, fatigue and solvent resistance. Besides, it is flammable but has low smoke emission.
PMMA usually behaves in a brittle manner when loaded, thus restricting its application. The solution to overcome is copolymerizes of MMA with other monomers, such as butyl acrylate (BA). Brandrup J. & Immergut E.H.’s study (as cited in Sirapanichart, S., Monvisade P., Siriphannon P., & Nukeaw J., 2011) found that poly(butyl acrylate), PBA is considered as a colourless transparent rubbery polymer at ambient temperature, thus it is commonly used in copolymer systems to alleviate the brittleness of the final product.
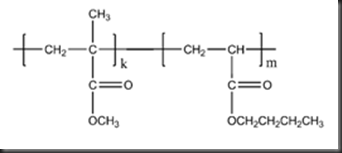
PMMA is produced through free radical polymerization from MMA. In this experiment, PMMA is synthesized via bulk polymerization by using free radical that produced by redox initiator. MMA is supplied with a small amount of inhibitors (an organic acid), which is used to prevent polymerization during shipping and storage. By using excess initiator, MMA still can be polymerized in the presence of inhibitors but low yield will be produced. To maximize the yield of polymer, the inhibitors are usually removed prior to use in order to generate long chain polymer at fast rate. The inhibitor can be removed through the process of distillation, chromatography or base extraction. For the copolymerization between MMA and BA, the synthesis is following the same method as polymerization of pure MMA. The figure 2 below shows the possible polymerization between BA and MMA monomers.
| Figure 2 Polymerization between BA and MMA monomers. |
Apparatus:
Pasteur pipette, large test tube, beaker, heater, Buchner funnel, watch glass, boiling tube
Materials:
Methyl methacylate (MMA), butyl acrylate (BA), N,N-dimethylaniline, benzoyl peroxide, boiling chip, methanol, acetone, alumina, 10% sodium hydroxide solution
Instruments:
Infrared spectrophotometer, differentiate scanning calorimeter
Procedures:
Removal of inhibitor by extraction
1. MMA and BA were purified by extraction with 10% sodium hydroxide solution.
Polymerization of monomers
1. 10ml of MMA was added with 1 drop of N,N-dimethylaniline and 0.1g benzoyl peroxide. The mixture was placed in boiling water bath.
2. At 3 minutes interval, 1 drop of aliquot was transferred into a test tube with methanol. Observation was recorded.
3. After 15 minutes, boiling tube was cooled and polymer was dissolved in 10-15ml of acetone.
4. While stirring vigorously, the polymer solution was poured into a beaker containing 80-100ml methanol.
5. Precipitated polymer was collected by vacuum filtration. The percent conversion was determined.
6. Steps 1-5 were repeated by using 65:35 of MMA: BA.
Characterization of polymers
1. 20% w/v solution of PMMA in acetone was prepared and was casted on a glass plate.
2. Clear film was obtained for IR and TGA analysis.
3. Procedures were repeated by using mixture of MMA and BA.
Results and calculation:
Part 1
Table 1.1: Observation of PMMA in methanol
| Observations | |
| 3rd minute | Aliquots turns solution to cloudy. |
| 6th minute | White precipitate is formed at the bottom and clear solution. |
| 9th minute | White precipitate is formed at the bottom and clear solution. |
| 12th minute | White precipitate is formed at the bottom and clear solution. |
| 15th minute | White precipitate is formed at the bottom and clear solution. |
Table 1.2: Weight of PMMA synthesized
| Weight of empty petri dish | 52.75g |
| Weight of (petri dish + poly(methyl methacrylate) PMMA) | 55.36g |
| Weight of poly(methyl methacrylate) PMMA | 2.61g |
Table 1.3: Significant peaks of PMMA in IR spectrum (Appendix I)
| Functional groups | Wavenumber, v (cm-1) |
| C=O stretch | 1727 |
| C-O stretch | 1152 |
Part 2
Table 2.1: Observation of co-poly(MMA/BA) in methanol
| Observations | |
| 3rd minute | Small amount of white precipitate is formed and clear solution. |
| 6th minute | More white precipitate is formed and clear solution. |
| 9th minute | Larger amount of white precipitate is formed and clear solution. |
| 12th minute | More and more white precipitate is formed and clear solution. |
| 15th minute | Largest amount of white precipitate is formed and clear solution. |