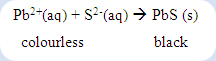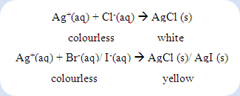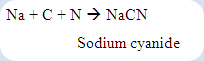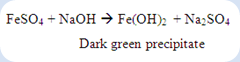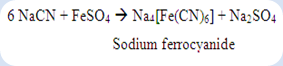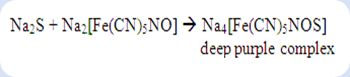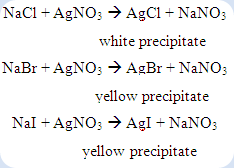Objective:
1. To carry out Lassaigne test in order to determine the elements (N,S halogens) present in the unknowns.
2. To identify the elements present in compounds and their colouration.
Introduction:
Qualitative analysis is always applied as a first step in identifying a compound when a new compound is readily prepared or isolated from some natural source. In an organic compound, elements carbon, hydrogen and oxygen are assumed to be present commonly. Nitrogen, sulphur and halogens (chlorine, bromine and iodine) may also present in the organic compound. The identification of elements in a given compound is a type of qualitative analysis since the experiment is dealing with the composition of a unknown compound. This experiment must be handled very carefully as further the analysis of the organic compound is according to the element present in it. Generally, the traditional technique is only can be applied to inorganic ions in aqueous solution.
When a new compound contains covalent bonding instead of ions, its molecule can be broken up into ions by controlled decomposition of the compound. The reagent used which cause the decomposition of the original unknown compound into the ions produced by the decomposition will reflect clearly those elements that were present initially in the compound. In this experiment, sodium fusion test (Lassaigne’s test) is used in elemental analysis of qualitative determination of elemental halogens, sulphur and nitrogen in a sample. Sodium is a very strong reducing agent that will able to break up the organic compounds carbon atom chain. It also will convert the atoms which are covalently bonded to the carbon chain to inorganic ions. The elements are detected by sodium fusion test. The organic compound is fused with metallic sodium to convert these elements into ionic mixture which dissolved in water and the filtrate is used to perform the tests.
The organic compound undergoes sodium fusion test which the carbon present in the particular compound is reduced partially to elemental carbon. The nitrogen present in the compound is reduced to cyanide ion, CN- while the sulphur present is converted into the sulfide ion, S2-. Any halogens (Cl, Br, I) that are present in the compound are reduced to the halides ions, Cl-, Br- and I-. A precipitate of an iron/cyanide complex with the characteristic of dark blue colour (Prussian blue) will be formed if cyanide ion is present in the compound. If sodium is not heated well, very little amount of cyanide ion is produced during fusion test. So, a greenish solid may result. It is always helpful to repeat the sodium fusion test in order to confirm the presence of nitrogen if only the greenish solid is being produced.
Several techniques can be used to test the presence of sulfide ion in the unknown organic compounds. Hydrogen sulfide gas will be produced if the filtrate obtained from sodium fusion products is acidified. This can be noted by the formation of lead(II) sulphate precipitate is formed after the lead(II) ions introduced into the solution.
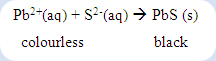
Besides, the sulfide ions can be tested by adding with sodium nitroprusside reagent. The formation of purple or violet colour shows the presence of sulfide ion. Silver ion reagent can be used to confirm the presence of halide ions in the compound. The formation of precipitate between halide ion and silver ion as shows below:
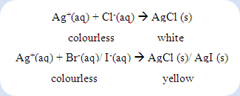
For the compound consists of more than one halogen, the mixed precipitate of silver halides can be observed as a result. However, it may be necessary to remove the cyanide ion and sulphide ion if they were present. Otherwise, these ions will precipitate with the silver ions to form precipitate which will mislead to the presence of halogens in the compound.
Apparatus:
Fusion tubes
Test tube
Evaporating dish
Gauze
Tongs
Materials
Sodium metal
Unknown organic compounds A,B,C
0.5M ferrous sulfate
0.5M ferric chloride
3M sulfuric acid
3M acetic acid
3M nitric acid
Sodium nitropusside
Lead acetate
0.1M silver nitrate
Procedure:
Part A: Sodium Fusion Test (Lassaigne)
1. 2-3 drops of unknown substance (for liquid) or 0.1g (for solid) was put into the fusion tube.
2. A piece of sodium was placed into the tube.
3. The tube was hold with a pairs of tong. The heating was gently to avoid spurting out of the sodium. When the sodium was molten, the compound was heated strongly until the end of the tube was red hot (continue heating for 1 minute).
4. The hot tube was plunged into 20ml of distilled water in a evaporating dish and covered with a gauze.
5. The tube was crushed with a pestle.
6. The mixture was filtered into a clean test tube. The colourless and clear filtrate was readily to be examined for the presence of nitrogen, sulphur, and halogens.
Part B: Test for nitrogen (Cyanide test)
1. To 1ml of filtrate, few drops of 0.5M ferrous sulfate was added and a dark green precipitate of iron(II) hydroxide is formed.
2. If sulphur is also present, precipitate will be black. More ferrous sulfate solution was added dropwise to precipitate all the sulphide ion until no more black precipitation is formed.
3. The mixture was heated to boiling with shaking, cooled and acidified with 3M sulfuric acid. If nitrogen is present, a blue colour (Prussian blue) appears immediately on addition of a trace of 0.5M ferric chloride solution.
Part C: Test for sulphur (sulphur test)
1. To 1ml of filtrate, a few drops of dilute solution of sodium nitroprusside were added. If sulphur ion is present, a deep purple colour will appear.
2. Alternatively, sulphide ion may be detected by precipitation as black lead(II) sulphide with lead(II) acetate solution which has been acidified by 3M acetic acid.
3. If halide is also present, white or yellow lead(II) sulphide precipitate may also formed.
Test for halogens
1. 1ml of filtrate was acidified with a few drops of 3M dilute nitric acid.
2. Silver nitrate solution was then added into the solution. Halides are indicated by the formation of a white or yellow precipitate.
Part E
The above procedure was repeated for unknown B and C. For each test, the observations were written down and the elements in the unknown were deduced.
Results:
Table 1 Observations of the unknown compounds on nitrogen test
| Unknown compound | Observation on nitrogen test | Inference |
| A | i) Some dark green precipitate and a little amount of dark precipitate were formed after brownish orange FeSO4 is added. ii) The precipitate become greenish after more FeSO4 is added. iii) The colour of precipitate remained unchanged after heating. iv) The precipitate does not change after sulphuric acid is added. v) Blue precipitate is formed immediately after adding of FeCl3 | Nitrogen is present. |
| B | i) The solution turns to yellow after brownish orange FeSO4 is added. ii) Brownish orange precipitate is formed after heated. iii) Colourless solution is formed after H2SO4 is added. iv) No change after FeCl3 is added. | Nitrogen is absent. |
| C | i) The solution turns to yellow after brownish orange FeSO4 is added. ii) Brownish orange precipitate is formed after heated. iii) Colourless solution is formed after H2SO4 is added. iv) No change after FeCl3 is added. | Nitrogen is absent. |
Table 2 Observations of the unknown compounds on sulphur test
| Unknown compound | Observation on sulphur test | Inference |
| A | a) Sodium nitroprusside i) A deep purple solution is formed immediately. b) Acidified lead(II) acetate i) A black precipitate is formed. | Sulphur is present. |
| B | a) Sodium nitroprusside i) A pale yellow solution is formed. b) Acidified lead(II) acetate i) A white precipitate is formed. | Sulphur is absent. |
| C | a) Sodium nitroprusside i) A pale yellow solution is formed. b)Acidified lead(II) acetate i) A white precipitate is formed. | Sulphur is absent. |
Table 3 Observations of the unknown compounds on halogens test
| Unknown compound | Observation on halogens test | Inference |
| A | Formation of white precipitate after nitric acid and silver nitrate are added. | Halogen is present. |
| B | Formation of white precipitate after nitric acid and silver nitrate are added. | Halogen is present. |
| C | Formation of yellow precipitate after nitric acid and silver nitrate are added. | Halogen is present. |
Discussion:
The organic compounds to be analyzed consist of basically of a chain of carbon atoms which various other atoms are attached. Since these elements are covalently bonded to the carbon chain, it is unable to dissolve in water to form cations and anions. However, sodium fusion test can be used to reduce those atoms that are covalently bonded to the carbon chain to inorganic soluble ions since sodium is a very strong reducing agent. In the Lassaigne’s test, the nitrogen can be reduced to form cyanide ions, CN-:
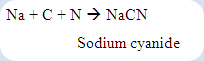
For sulphur, it had been reduced to form sulfide ion, S2- in Lassaigne’s test as shown in the following:

If both nitrogen and sulphur are present in the organic compound at the same time, then the chemical reaction below will take place in the test:

If halogens (Cl, Br, I) are present in the compound, the halogens will be reduced to form halide ions (Cl-, Br-, I-) during the sodium fusion test.

The inorganic ions in aqueous solution could be easily observed after undergo certain tests which can indicates the presence of elements in the particular compounds.
In the cyanide test, the filtrate of compound A was added with ferrous sulfate, a dark green precipitate was formed. The formation of ferrous hydroxide was produced from the reaction between ferrous sulfate and sodium hydroxide.
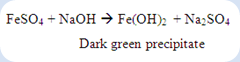
The sodium hydroxide was formed by the reaction of unreacted sodium metal with water due to incomplete reaction of sodium fusion with compound A.

The FeSO4 solution was added to confirm the presence of NaOH and to react completely with it in the filtrate. At the same time, a small amount of black precipitate was formed at the bottom but it was disappeared after more ferrous sulphate was added. The formation of black precipitate may be due to the ferrous sulphide exists in the mixture.

The equation below shows that the ferrous sulphate was reacted with the sodium cyanide to form sodium ferrocyanide as the main product.
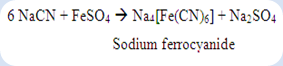
The sulphuric acid and increase in temperature was used to increase the suitable medium for the formation of complex. As a result, ferric-ferrocyanide complex with the colour of Prussian blue was precipitated out after ferric chloride is added to oxidize the Fe2+ to become Fe3+. This Prussian blue precipitate indicates that the unknown A contains nitrogen in the compound.

Some of the Fe3+ was formed before the oxidation of ferric chloride. This might be due to the air oxidation of iron(II) ions in the mixture before the ferric chloride is added. For compounds B and C, a negative result is obtained which end up with colourless solution as results. Hence, these shown nitrogen are absent in the both organic compounds.
The reduced sulfide ion can be confirmed by using two different tests which were sodium nitroprusside test and lead(II) acetate test. For the first test, the appearance of deep purple solution shows the positive result. The formation of sodium sulphonitroprusside is a complex that was formed between the sodium nitroprusside and sodium sulphide.
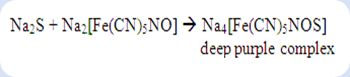
In another test, the black precipitate will be formed if the sulphur is present in the compound. The formation of black precipitate shows a positive result for this test.

The compounds B and C did not consisting sulphur in their structure because they cannot give the positive result on both tests. A pale yellow solution was formed after sodium nitroprusside was introduced and the solution shows the colour of sodium nitroprusside. In the latter test, white precipitate was formed may be due to the precipitation of the lead (II) ions with the halide ions. Based on the observation, the organic compound A containing sulphur while compound B and C did not containing sulphur.
In the halogens test, if white or yellow precipitation takes place after silver nitrate was added into the filtrate from compound A, B and, C respectively is known as the positive result. If cyanide ion or sulfide ion present in the compound, the acidified solution must be heated until boiled in order to expel the hydrogen cyanide gas and hydrogen sulfide gas. This step was taken to avoid the cyanide ions and sulfide ions cause the error in the halogens test. The sodium halide formed during the sodium fusion test was reacted with the silver nitrate to form the insoluble silver halide as precipitate in the solution.
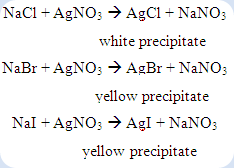
The white precipitate was formed in the filtrate from compound A while yellow precipitate were formed in the both filtrate from the compound B and C. This is mean that the halogen presents in the compound A probably is chlorine whereas the halogens exist in the compound B and C were possibly bromine or iodine.
Precaution steps:
1. Safety glasses should be worn in the laboratory at all the time.
2. Do not add too much of water into the sodium fusion products unless if has been established with certainly that all elemental sodium has been destroyed.
3. The fusion of unknown compound is carried out in the fume hood.
4. When heating the liquids, use a small flame instead of large and move the test tube quickly through the flame.
5. The heating process of unknown compound must carried out in fume hood.
6. Avoid the corrosive sulfuric, nitric and acetic acids touch on skin because it can be dangerous to skin.
 Monomer of high density polyethylene
Monomer of high density polyethylene  Polymer of high density polyethylene
Polymer of high density polyethylene