Objective:
Part I:
1. To learn the technique of TLC and the visualization of colourless components.
2. To identify an unknown drug by a TLC comparison with standard compounds.
Part II:
1. To learn the technique of column chromatography.
2. To separate the mixture of pyrene and p-nitoraniline by column chromatography.
Introduction:
Chromatography is a common laboratory technique to separate and analyze two or more analytes in the mixture by distribution of two phases: a stationary phase and a mobile phase. The stationary phase is a phase which allows the mobile phase to travel along. These two phases can be solid-liquid, liquid-liquid or gas liquid. This method works on the principle that different compounds with different solubilities and adsorptions to the two phases which they are to be partitioned. The compounds to be retained on the stationary phase are more interacted with it while the compounds to be moving carried along by the mobile phase. The rates of migration for each component on the system is depends on the degree of the compounds of mixture are adsorbed by the stationary phase and their degree of solubilities on the mobile phase. The stronger the adsorption by stationary phase, the slower the compounds travels along the mobile phase. As a result, the distance of separation for each compound in the mixture will be different. The types of chromatography is divided into few types which include gas chromatography(GC), high performance liquid chromatography(HPLC), thin layer chromatography(TLC), and column chromatography(CC). However, only TLC and CC are applied in this experiment.
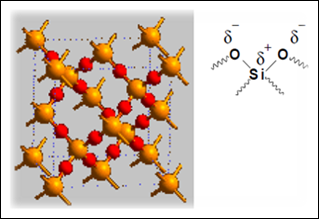
Thin layer chromatography (TLC) is a solid-liquid form of chromatography where the stationary phase is usually a polar adsorbent while the mobile phase can be one single solvent or a combination of solvents. TLC is a quick and inexpensive technique that can be used to 1) determine the number of compounds in a mixture, 2) identify the compounds, 3) monitor the progress of a reaction, 4) determine the effectiveness of a purification, 5) determine the appropriate conditions for column chromatography separation, and 6) analyze the fractions obtained from column chromatography. In thin layer chromatography, the stationary phase is refers to polar adsorbent, usually is silica gel or aluminium oxide which is coated on an aluminium plate. Generally, polar solvent is used as the mobile phase in the system to carry the analytes by passing through the stationary phase. The stationary phase in TLC chromatography is typically silica gel, (SiO2.xH2O)n which has been shown in the diagram 1 below:
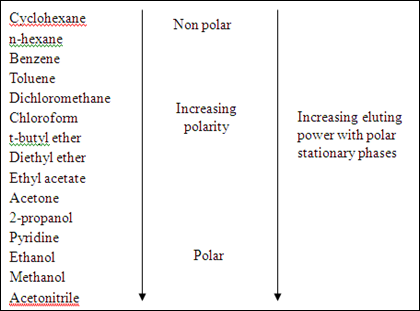
Different compounds are spotted on the silica gel plate (stationary phase), the prepared solvent (mobile phase) will carry the compounds goes up along the plate through capillary action which the solvent travels from the bottom to the solvent front. Once the dilute solutions of compounds are spotted on the plate, then development of solvent is the next. After that, the position of each compounds can be visualized under the presence of ultraviolet (UV) light. The compounds in the mobile phase will have different interaction with the polar stationary phase. The factors are mainly depends on the polarity of adsorbent (silica gel in this experiment), solvent polarities, and functional groups of the compounds. The polar adsorbent will more strongly attract the polar molecules of compounds and it will have lower affinity to the non-polar compounds. Hence, the movement of compounds with different polarities could be different. In addition, the polarity of solvent is very important to the compound separations, a solvent system may increase in its polarity by changing the composition of the solvent mixture. The more polar the solvent, the faster the compounds can be drawn up, which means the further the compounds move. The comparison of the polarities of solvent are listed down in the diagram 2.
The second factor that affects the interaction between stationary phase and compounds is functional group of each compound. The highly polar groups in compound will cause the stronger adsorption and eluted less readily to the stationary phase compared to less polar compounds. Hence, the highly polar compounds will tend to interact strongly with the polar adsorbents and absorb onto the fine particles of the absorbent, hence it cannot travel further. The adsorption strengths of each compound having the following types of functional groups in the order of increasing group polarities.
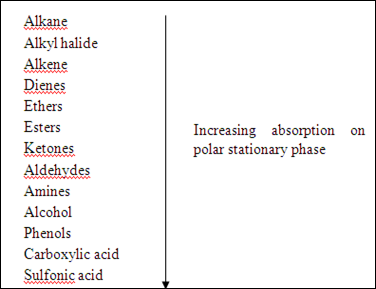 However, the variation may take place which depends on the overall structure of each compound.
However, the variation may take place which depends on the overall structure of each compound.
Column chromatography is one of the most useful methods for the separation and purification of both solids and liquids when carrying out small-scale experiments. Like TLC, the silica gel is used as a stationary phase while an organic solvent is used as the mobile phase which its polarity should be lower than silica gel. Column chromatography is carried out in a glass tube that is clamped vertically with the mixture of samples at the top. The samples are dissolved in a small quantity of solvent which is used to apply on the top of the vertical column. In this case, the solvent (mobile phase) will tend to flow down through stationary phase (silica gel) instead of the capillary action. The compound with less polar characteristics will elute faster through the column due to the silica gel has the strong affinity towards the more polar compounds. Eventually, the compounds start to be separated as the solvent is allowed to flow through the stationary phase. Due to the difference in their polarities, the solvent acts as the mobile phase will carry the less polar compounds further down from the top in the system. Below the column, several flasks are used to collect the solvent with compound in various fractions. The solvent is continually added to the top of the column until each band resolves and is carefully collected. As a result, the experiment is end up with the separation of two compounds from the mixture into two different portions. With coloured substances, the bands might be visible and easily to be collected as they run off the column. However, colourless compounds can be observed directly. So, the particular compound in eluting solvent is collected in many small fractions and testing each of them by using TLC. A fresh solvent (mixture of solvent similar to the eluent in column chromatography) is being used in TLC for the next step of identifying the compounds.
The total distance traveled by the compounds on silica gel plate are measured and is being compared to each other. The migration rate of each compound is compared by using the retardation factor, Rf. Retardation factor is the ratio of distance traveled of the compound to the distance of the solvent traveled. Retardation factor, Rf is the distance of compound traveled divided by the total distance of solvent travelled in TLC plate. If two spots in the TLC plate travel the same distance or have the same value of Rf, then both compounds might be concluded as the same compounds.
Part I: TLC analysis on analgesics drugs
Apparatus: UV lamp, capillary tube, 250ml beaker
Materials: aspirin, acetaminophen, caffeine, unknown A, unknown B, TLC plates, ethyl acetate, hexane, iodine
Procedure:
PartA: Spotting the TLC plate
1. A TLC plate was obtained from instructor. Holding the edges of the plate carefully.
2. Set the plate down on a clean and dry surface, then a line was drawn across the plate about 1.0cm from the bottom of the plate by using a 2B pencil.
3. Five lines of 2-3mm were drawn, spaced about 0.6cm apart and running perpendicularly through the lines across the bottom of the TLC plate. 0.5cm must be spaced from each side of the edges.
4. 5 different analgesics were spotted on each 2-3mm lines. Firstly, acetaminophen was spotted on the plate, followed by caffeine, unknown A, aspirin and lastly the unknown B. The plate was examined under the UV light to check whether enough each solution has been applied.
Part B: Developing the TLC plate
1. A developing chamber was prepared by using a 250ml beaker, a half-piece of filter paper inside and aluminium foil to cover.
2. Mixture of 1:3 of ethyl acetate : hexane was poured into the beaker to the depth of about 1cm. The TLC plate was placed in the developing chamber.
3. After the solvent has risen to near the top of the plate, the plate was removed.
Part C: Visualization
1. The colourless compounds were visualized by illumination of the plate with UV lamp.
2. The spots were outlined by using a 2B pencil. The spots may be visualized by putting the plate in an iodine chamber for a couples of minutes.
Part D: Comparison of the unknown with reference standards
1. The plate was sketched in notebook and the Rf value was calculated for each spot.
2. The unknown drug was determined based on Rf value.
Part II: The separation of pyrene and p-nitroaniline by column chromatography
Apparatus: glass column, UV lamp, capillary tube, 250ml beaker, test tubes, glass funnel
Materials: pyrene, p-nitroaniline, TLC plates, ethyl acetate, hexane, iodine
Procedure:
Part A: Column preparation
1. A 49cm chromatography column, 15g of deactivated Silica gel and 110ml of developing solvent mixture (ethyl acetate:hexane; 1:3) were obtained.
2. A slurry of the adsorbent( silica gel) was prepared with a solvent in a 250ml Erlenmeyer flask.
3. A small plug of cotton was pushed into the constriction at the bottom of the column. The column was clamped in a vertical position and 0.5cm layer of the sodium sulfate anhydrous was added on top of the cotton.
4. Ensuring the stopcock of the column is closed, 15ml of solvent was poured in. After setting, all the slurry was quickly decanted through a funnel into the column.
5. The stopcock was opened and allowed the solvent to drain while tapping the walls of the column with the ends of a folded price of rubber tubing.
6. Once the solvent level is within 6cm of the top of the adsorbent, the packing should be essentially complete. 0.5cm level layer of sodium sulfate anhydrous was added on the adsorbent.
7. Excess solvent was drain off until its level is precisely on top of the sodium sulfate anhydrous and the stopcock was closed.
Part B: Separation and collection of pyrene and p-nitroaniline
1. The mixture of pyrene and p-nitroaniline was took and a few drops of ethyl acetate was added to dissolve as much as possible.
2. The solution was transferred directly to the top of the sulfate anhydrous layer with a dropper.
3. The solvent was drain off until the mixture solution is just below the top of the sodium sulfate anhydrous.
4. The wall was rinse with 1ml of fresh solvent(ethyl acetate/hexane 1:3) and was drain until the level was once again below the top of sodium sulfate anhydrous. The rinsing of the walls was repeated until the solvent above the silica gel is virtually colourless.
5. The column was filled carefully with the fresh solvent(ethyl acetate/hexane 1:3) and allowed solvent to drain.
6. The separation of bands was observed as the column develops. The colourless band of pyrene was collected into 3 test tubes.
7. When the edge of the yellow band (p-nitroaniline) reached the lower part of column, a new test tube was replaced and the yellow band was collected into three fractions.
8. Each fraction was concentrated to a small volume by evaporation for analysis by TLC.
Part C: Analysis of the fraction
1. The fractions were spotted on a TLC silica gel plate along with the reference pyrene and p-nitroaniline.
2. The TLC plate was developed in a developing chamber containing a mixture of ethyl acetate/hexane 1:3.
3. The TLC plate was visualized with the UV lamp to determine the fraction of pure pyrene and pure p-nitroaniline.
4. The chromatogram was drawn in the notebook.
5. The Rf value was calculated for pyrene and p-nitroaniline.
6. The pure fraction of pyrene was combined in a pre-weigh test tube and the pure fraction of p-nitroaniline in another test tube. Both solvents were evaporated on a stem bath.
7. Once the solvent has evaporated, the weight of the pure pyrene and p-nitroaniline were calculated.
Results:
Part I:
Total distance of solvent travelled from bottom line in TLC plate = 8.0cm
Retardation factor, Rf
| Samples | Distance travelled from the bottom line (cm) | Retardation factor, Rf |
| Acetaminophen | 0.50cm | 0.0625 |
| Caffeine | 0.35cm | 0.0438 |
| Unknown A | 0.50cm | 0.0625 |
| Aspirin | 2.40cm | 0.3344 |
| Unknown B | 2.20cm | 0.2750 |
Diagram of acetaminophen, caffeine, unknown A, aspirin, and unknown B travelled on the TLC plate
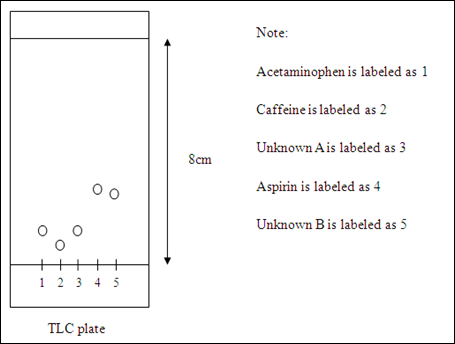 Inference: unknown A is acetaminophen whereas the unknown B is aspirin.
Inference: unknown A is acetaminophen whereas the unknown B is aspirin.
Part II:
Total distance of solvent travelled from bottom line in TLC plate = 8.0cm
Retardation factor, Rf
| Samples | Distance travelled from the bottom line (cm) | Retardation factor, Rf |
| 1st fraction of pyrene | 6.1cm | 0.7625 |
| 2nd fraction of pyrene | 6.1cm | 0.7625 |
| 3rd fraction of pyrene | 6.1cm | 0.7625 |
| 4th fraction of pyrene | 6.1cm | 0.7625 |
| 1st fraction of p-notroaniline | 1.6cm | 0.2000 |
| 2nd fraction of p-notroaniline | 1.7cm | 0.2175 |
| 3rd fraction of p-notroaniline | 1.7cm | 0.2175 |
| 4th fraction of p-notroaniline | 1.7cm | 0.2175 |
Diagram of pyrene and p-nitoraniline travelled on the TLC plate
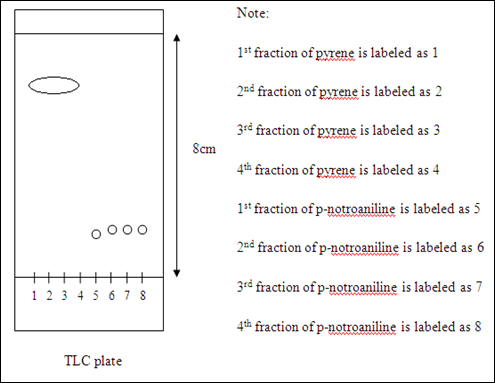 Inference: pyrene is present in the 1st to 4th spots while 5th to 8th spots containing p-nitroaniline.
Inference: pyrene is present in the 1st to 4th spots while 5th to 8th spots containing p-nitroaniline.
Discussion:
In the thin layer chromatography, the eluent (solvent) is prepared by using a mixture of hexane and ethyl acetate in the ratio of 3:1. The polarity of the particular solvent cannot be too low because the polar compounds will not be able to carry by the eluent and will not be separated, so that the separation might not be observable. If the solvent of too high polarity is used, the polar compound will travel so fast that the separation between non-polar compound and polar compound to become so small and poor separation will be observed. The solvent mixture of ethyl acetate and hexane (1:3) is believed that it has the optimized solubility for the organic compounds to dissolve in the solvent. In another word, the compounds can be easily to be carried by the solvent in the TLC plate. A few drops of acetic acid were added into the particular solvent in order to protonate the organic compound on the TLC plate and prevent them from ionization. This is due to the deprotonation of organic compounds will cause the compound to form ions. So, the adding of acetic acid is used to maintain the structure of organic compound as they can travel up through the TLC plate. Before the TLC plate is placed into the solvent. A filter paper was dipped inside the solvent in a beaker which is covered by using an aluminium foil. This is to create a system that prevents the vapourization of organic solvent and hence the solvent is allowed to travel up along the plate faster. After the TLC plate was introduced into the solvent, the solvent is starting to migrate itself and the compounds on the TLC plate until the solvent front has been reached.
There are three components in the TLC which include the TLC plate with adsorbent, the development solvent and the organic compounds that to be analyzed. The adsorbent, silica gel consists of a three dimensional network of thousands of alternating silicon and oxygen bonds. It is a very polar and is capable of hydrogen bonding due to its partial positive charge in silicon and partial negative in oxygen. The silica gel with compete with the development solvent for the organic compounds as the solvent is traveling up through the TLC plate. The silica gel tends to bind the compounds (on stationary phase) while the development solvent tried to dissolve the compounds (on mobile phase) in order to carry the compounds along the plate as the solvent travels up. All the compounds are possible to be adsorbed into the stationary phase however the time of adsorption of compounds in the particular phase is depends on the polarity of each compound. The more polar the compound is, the longer the time taken that the compound adsorbed into the stationary phase so it eluting speed is slower (more time on stationary phase). Less polar compounds are weakly adsorbed, so the time taken for less polar compounds to be adsorbed on stationary phase is shorter. As a result, the less polar compounds can travel further along the plate compared to the more polar compounds.
In this experiment, the analgesics drugs have been analyzed by using TLC are acetaminophen, caffeine, and aspirin. The structure of each compounds are shown as below:
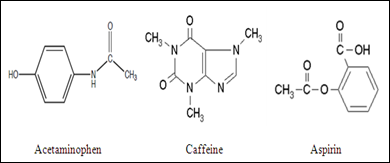 The polarity of the compounds could be compared by looking at the structure of these compounds. The polarity of the compound is due to the effect of electronegativity in atoms and the asymmetrical structure of compound. The unequal sharing of electron within the bond causes the formation of an electric dipole which leads to partial positive and partial negative exist in the several atoms. The highly electronegative atoms present in these compounds are O, N, and F. These highly electronegative atoms tend to withdraw the electrons towards themselves from the aromatic ring and hence it polarizes the compounds. Nitrogen and oxygen atom present in these compounds have higher electronegativity compared to the carbon atom and hydrogen atom. Thus, nitrogen and oxygen atom acquire partial negative charge while the carbon and hydrogen atom acquire partial positive charge. Hence, caffeine has the highest polarity, followed by acetaminophen while aspirin possesses the lowest polarity. The sequence of increasing in polarity is arranged in the order below:
The polarity of the compounds could be compared by looking at the structure of these compounds. The polarity of the compound is due to the effect of electronegativity in atoms and the asymmetrical structure of compound. The unequal sharing of electron within the bond causes the formation of an electric dipole which leads to partial positive and partial negative exist in the several atoms. The highly electronegative atoms present in these compounds are O, N, and F. These highly electronegative atoms tend to withdraw the electrons towards themselves from the aromatic ring and hence it polarizes the compounds. Nitrogen and oxygen atom present in these compounds have higher electronegativity compared to the carbon atom and hydrogen atom. Thus, nitrogen and oxygen atom acquire partial negative charge while the carbon and hydrogen atom acquire partial positive charge. Hence, caffeine has the highest polarity, followed by acetaminophen while aspirin possesses the lowest polarity. The sequence of increasing in polarity is arranged in the order below:
Based on the retardation factor, Rf, the polarities of these compounds can be compared. The higher the Rf, the lower the polarity and hence the distance traveled by the compound would be longer. Aspirin has the highest Rf value. The interaction between aspirin and stationary phase (silica gel) is the weakest which allows the aspirin can travels up along the plate fastest. A stronger interaction in between the silica gel and acetaminophen causes the compound move slower when travels up the TLC plate. The polarity of the acetaminophen is considered lower than caffeine but the difference is quite small. This can be shown in the distance traveled by both compounds which corresponding to their Rf value. In this part, TLC method can be used to identify the two unknowns spotted on the TLC plate. The compounds with same polarities usually travel up through the plate in the same distance and possess the same Rf value provided the stationary phase and mobile phase are identical. Unknown A is predicted as acetaminophen while unknown B is forecasted as the aspirin. This is because the Rf value of unknown A and unknown B are same with the Rf value of acetaminophen and aspirin respectively.
Column chromatography is used to purify the individual organic compound from the mixture of compounds. In column chromatography, the stationary phase and mobile phase used are same as used in thin layer chromatography. The adsorbent (stationary phase) used is a solid which silica gel is usually being used. The eluent (mobile phase) used is the mixture of hexane and ethyl acetate in the ratio of 1:2. The compounds used were the pyrene and p-nitroaniline. The structure of each compound are shown as below: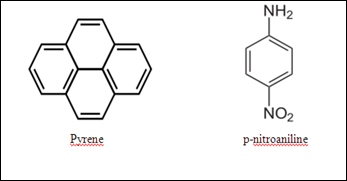 Based on the structure of the compound, the pyrene has the lower polarity due to its delocalized electron in the aromatic ring. The delocalizing π electrons are distributed evenly in the whole structure and hence it stabilize the pyrene. In addition, there is no other electronegative atom attach to the pyrene as substituent, so the electron are just delocalize within the four aromatic ring of pyrene. On the other hand, the p-nitroaniline has higher polarity due to its electronegative substituent in the structure. N and O atoms are the highly electronegative atoms which tend to withdraw the electrons from the benzene ring towards the atoms. This causes the arisen of partial positive charge and partial negative charge in the substituent and within the benzene ring. Consequently, the structure of p-nitroaniline induces the polar property of the compound and hence the polarity of p-nitroaniline is much more higher than pyrene. Due to the difference in polarities in each compound, the interaction with the silica gel would be different. The polar compound would have the stronger interaction with the silica gel. This is because the polar silica gel tends to pull the polar p-nitroaniline toward the stationary site and causes the compound becomes more difficult carried by the solvent through the system. As a comparison, the less polar pyrene was weakly adsorbed into the stationary phase. The silica gel would not tend to attract the less polar compound into the stationary site and hence the particular compound can be carried by the solvent to reach the bottom of the column. The pyrene is said that it elute faster than the more polar p-nitroaniline.
Based on the structure of the compound, the pyrene has the lower polarity due to its delocalized electron in the aromatic ring. The delocalizing π electrons are distributed evenly in the whole structure and hence it stabilize the pyrene. In addition, there is no other electronegative atom attach to the pyrene as substituent, so the electron are just delocalize within the four aromatic ring of pyrene. On the other hand, the p-nitroaniline has higher polarity due to its electronegative substituent in the structure. N and O atoms are the highly electronegative atoms which tend to withdraw the electrons from the benzene ring towards the atoms. This causes the arisen of partial positive charge and partial negative charge in the substituent and within the benzene ring. Consequently, the structure of p-nitroaniline induces the polar property of the compound and hence the polarity of p-nitroaniline is much more higher than pyrene. Due to the difference in polarities in each compound, the interaction with the silica gel would be different. The polar compound would have the stronger interaction with the silica gel. This is because the polar silica gel tends to pull the polar p-nitroaniline toward the stationary site and causes the compound becomes more difficult carried by the solvent through the system. As a comparison, the less polar pyrene was weakly adsorbed into the stationary phase. The silica gel would not tend to attract the less polar compound into the stationary site and hence the particular compound can be carried by the solvent to reach the bottom of the column. The pyrene is said that it elute faster than the more polar p-nitroaniline.
The total number of fraction have been collected was 19 fractions after all the yellow bands (p-nitroaniline) transferred out from the column. After evaporated most of the solvent, the 19 fractions were concentrated into eight fractions which each of the fraction have been used to apply on a TLC plate by using TLC. The separation of compounds is considered as successful since the 1st to 4th spots are containing pyrene only. The 5th to 8th spots are only contain the compound of p-nitroaniline. These can be proved by checking the distance of compounds traveled on TLC plate of each fraction and the corresponding to the Rf value are the same. However, the colours of the 1st and 4th fractions of pyreme on the TLC plate were faded compared to the 2nd and 3rd fractions. This might be due to the concentration of pyrene in the former fractions were not the same with the latter fractions. The amount of pyrene collected in the 1st fraction is less because it collected more solvent. The 4th fraction also has less concentration of compound because the concentration left in the column after 2nd and 3rd fractions of compound have been collected. The four spotted compounds were noticed that they were connected to each other on the TLC plate. This might be due to the diameter of the spotted place was too big. The 1st and 4th fractions of p-nitroaniline also have the same condition which their colours on the TLC plate were faded. It was believed that the 1st fraction and 4th fraction of p-nitroaniline have the similar condition with the 1st and 4th fractions of pyrene. The concentrations of the p-nitronaniline were not enough so they appear lighter colours on the plate under the sources of ultraviolet light.
By using the iodine as the development solvent in TLC, the similar observation for the both pyrene and p-nitroaniline were observed. The distance traveled of compounds were almost similar compared to previous solvent. But, the difference was just the colour of the spots on the TLC plate.
Precaution steps:
1. Do not move the beaker after the TLC plate had introduced into the beaker.
2. Do not look directly to the ultraviolet lamp.
3. Avoid to use pencil on the TLC plate
4. Do not touch on the surface of silica gel of TLC plate by using finger.
Thin layer chromatography can be used to observe the progress of a reaction, identify compounds present in a given mixture, and determine the purity of a substance. TLC method is simplest, easy and quick results given to the chemist as how many compounds are purify from the mixture. TLC is used in organic chemistry, a method of chemistry to identifying compounds.
ReplyDeleteAwesome
ReplyDeleteHow if TLC plate shows paracetamol is more polar than caffeine? How am I able to explain this? What is the contributing factor for this result?
ReplyDelete